- Journal List
- GeroScience
- v.42(3); 2020 Jun
- PMC7287009

Long-term treatment with spermidine increases health span of middle-aged Sprague-Dawley male rats
 4,5,6
4,5,6Madalina Filfan
1Doctoral School, University of Medicine and Pharmacy, Craiova, Romania
Andrei Olaru
2Department of Ophtalmology, University of Medicine and Pharmacy, Craiova, Romania
Ion Udristoiu
3Department of Psychiatry, University of Medicine and Pharmacy, Craiova, Romania
Claudiu Margaritescu
4Department of Pathology, University of Medicine and Pharmacy, Craiova, Romania
Eugen Petcu
5Gold Coast Campus and Queensland Eye Institute, Griffith University Menzies Health Institute of Queensland, QLD, Brisbane, 4000 Australia
Dirk M Hermann
6Chair of Vascular Neurology, Dementia and Ageing Research, Department of Neurology, University Hospital Essen, Germany, University of Duisburg-Essen, Duisburg, Germany
Aurel Popa-Wagner
4Department of Pathology, University of Medicine and Pharmacy, Craiova, Romania
5Gold Coast Campus and Queensland Eye Institute, Griffith University Menzies Health Institute of Queensland, QLD, Brisbane, 4000 Australia
6Chair of Vascular Neurology, Dementia and Ageing Research, Department of Neurology, University Hospital Essen, Germany, University of Duisburg-Essen, Duisburg, Germany
 Corresponding author.
Corresponding author.Abstract
Let alone calorie restriction, life span extension in higher organisms has proven to be difficult to achieve using simple drugs. Previous studies have shown that the polyamine spermidine increased the maximum life span in C. elegans and the median life span in mice. However, younger subjects (< 40 years of age) are infrequently prescribed nor self-medicating with antiaging drugs. Therefore, in the present study, we aimed at assessing the effect of long-term treatment with spermidine given in the drinking water on behavioral performance and longevity of male, middle-aged Sprague-Dawley rats. We report that spermidine given in the drinking water did not extend neither the median nor the maximum life span of the middle-aged male Sprague-Dawley rats. However, spermidine treatment had a beneficial effect on the body weight and the kidney tubules, liver, and heart morphology. Behaviorally, spermidine led to a reduction in anxiety and an increase in curiosity, as assessed by exploratory behavior. Moreover, long-term treatment with spermidine enhanced autophagy in the brain and led to a diminished expression of the inflammatory markers, Tgfb, CD11b, Fcgr1, Stat1, CR3, and GFAP mRNAs in several cortical region and hippocampus of the treated rats suggesting that one beneficial effect of the long-term treatment with spermidine is an attenuated proinflammatory state in the aged brain. Our results suggest that long-term treatment with spermidine increases health span of middle-aged rats by attenuating neuroinflammation and improving anxiety and exploratory behavior.
Introduction
As societies age, researchers worldwide are interested in finding antiaging treatments to enable aged people to live a longer life in good health. Most of the studies aimed at manipulating the life span have been done in lower organisms. Thus, short-term calorie restriction achieved by intermittent fasting during early life induced life span expansion in Drosophila by 33%, most likely by a TOR-independent mechanism (Catterson et al. 2018). However, most of studies were concerned with the beneficial effects on life span of antioxidants and anti-inflammatory dietary supplements. Thus, exposing Drosophila melanogaster to resveratrol orally resulted in prolonged life span possibly via a reduction in the oxidative stress (Abolaji et al. 2018) while Drosophila flies kept on a diet supplemented with an extract of Ilex paraguariensis also displayed an extended life span by 13% (Niraula et al. 2018). In another study, the antioxidant fucoxanthin was used to extend life span by 33% in both Drosophila melanogaster and Caenorhabditis elegans albeit at the expense of decreased flies fecundity (Lashmanova et al. 2015). Similarly, Drosophila melanogaster treated with nonsteroidal anti-inflammatory drugs displayed increased life span but decreased fecundity (Danilov et al. 2015).
Life span manipulations in the worm Caenorhabditis elegans were also at the focus of several studies. Thus, the anticonvulsant drug, ethosuximide, was used to prolong life span in C. elegans by inhibiting the function of specific chemosensory neurons (Collins et al. 2008). Similarly, the ACE inhibitor, captopril, was used by Kumar and colleagues to extend the mean adult life span by 23% and maximal adult life span by 18% most likely via inhibiting the expression of the homolog of human ACE in this worm, acn-1 (Kumar et al. 2016).
Inhibition of mTOR (target of rapamycin) signaling using rapamycin has been shown to extend life span in C. elegans (Vellai et al. 2003; Jia et al. 2004) and Drosophila melanogaster (Kapahi et al. 2004) and increased life expectancy and health span in mice (Anisimov et al. 2010; Harrison et al. 2009; Miller et al. 2011).
Mouse-sized naked mole-rats (Heterocephalus glaber), which is expected to live 6 years on the basis of allometry, do not conform to Gompertzian laws of age-related mortality. In the search for metabolic biomarkers of slow and successful aging phenotypes, it was recently reported on low circulating levels of the amino acids linked to the methionine pathway, which concur with metabolome reports on the long-lived Ames dwarf mice and methionine-restricted rats, calorically restricted mice, as well as on those observed in hibernating ground squirrels (Lewis et al. 2018).
Autophagy is a major pathway for the turnover of organelles and thus the rejuvenation of the cell. Therefore, efficient extension of life span by genetic, pharmacological, and dietary manipulations may require autophagy (Madeo et al. 2018; López-Otín et al. 2016; Madeo et al. 2015; Rubinsztein et al. 2011). One drug that has been shown to extend longevity by enhancing autophagy is the polyamine spermidine which has been shown to increase mean and maximal longevity in yeast, worms, flies, and human immune cells (Eisenberg et al. 2009).
Polyamines are considered important for survival, being involved in multiple pathways and biological processes, while abnormal changes in polyamine levels are associated with diseases and aging (Hussain et al. 2011). Thus, an analysis of polyamine levels in female mice aged 3, 10, and 26 weeks old revealed that spermidine concentration decreased with age in 11 from 14 tissues and spermine decreased only in the skin, muscles, and heart, while putrescine was decreased in all the 14 tissues and ages (Nishimura et al. 2006). Mice fed with a polyamine-rich food containing spermine, spermidine, and putrescine increased survival at 22 months (Soda et al. 2009).
More recently, administration of the polyamines spermine and spermidine in drinking water extended the median life span of young mice as compared to that of controls (Eisenberg et al. 2016). However, younger subjects (< 40 years of age) are infrequently prescribed nor self-medicating with antiaging drugs. Therefore, in the present study we aimed at assessing the effect of long-term treatment with spermidine given in the drinking water on behavioral performance and longevity of male, middle-aged Sprague-Dawley rats.
Materials and methods
Animals and treatment
Middle-aged (18 months) male Sprague-Dawley rats were maintained on a 12-h light/dark cycle and 23 °C and allowed free access to food and water. The rats were randomly assigned to two groups: (1) control group (N = 45) and (2) treatment group (N = 45). The rats were treated with 25 mg/kg/day spermidine (Sigma-Aldrich, Munich, Germany) dissolved in the drinking water, until death. The control group received water only. Survival was daily controlled. All studies with use of laboratory animals were being performed in accordance with Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes with relevant acts and regulations. All protocols were approved by the local animal ethics committee, 112-14112018. All appropriate measures were taken to minimize pain and suffering.
Behavioral tests and histological analysis
Rats were subjected every 2 weeks to a variety of somatosensory, motor, learning, and memory tests and in total for 16 weeks. All testing was performed from 2 to 5 PM by an experimenter who was blinded to the group identity.
Elevated plus maze
The elevated plus maze (EPM) is a test measuring anxiety in rodents. The model is based on the test animal’s aversion to open spaces and tendency to be thigmotaxic. In the EPM, this anxiety is expressed by the animal spending more time in the enclosed arms. For the test, we used an elevated, plus-shaped (+) apparatus with two open and two enclosed arms. Briefly, rats are placed at the junction of the four arms of the maze, facing an open arm, and entries/duration in each arm are recorded by video tracking for 5 min.
Exploratory activity in a small three-dimensional environment: cylinder test
The exploratory behavior of animals was measured using the cylinder test (Gharbawie et al. 2004). Briefly, each animal was individually placed in the cylinder and filmed for 5 min. From the video records of their behavior, activity on the vertical surface of the cylinder was analyzed. Exploratory behavior was organized into bouts lasting one to 2 min. The number of touches with the forelimbs when exploring the wall of the cylinder while it maintained contact with the floor only with its hindlimbs was taken as a measure of exploratory activity.
Bilateral sensorimotor coordination: rotating beam walking test
The rotating beam task assesses coordination and sensorimotor function. Each rat was tested for its ability to cross a rotating (6 rpm) horizontal rod. The task involved both forelimb and hindlimb coordination and the fine vestibulo-motor function. The performance was videotaped from above, and recordings were analyzed using a slow-motion video as previously described by us. The score assessment was done as previously described (Buchhold et al. 2007). Briefly, each rat was tested for its ability to negotiate a rotating (6 rpm) horizontal rod. Normally, the rats will cross the cylinder with their feet squarely on the top surface of the rod. Using either a forelimb or hindlimb on the side of the beam was considered to be a fault. The time taken for the rat to traverse the rotating cylinder and join a group of rats visible at the finish line was measured. The score assessment was twofold: (1) time (seconds) required to traverse the rotating cylinder and (2) the score as follows: 0, rat falls immediately (onto a soft surface); 1, rat does not walk forward, but stays on the rotarod; 2, rat walks but falls before reaching the goal; 3, rat traverses the rod successfully, but the limbs are used asymmetrically; 4, the left hindlimb is used less than 50% of the time taken to traverse the rod; 5, the rat successfully traverses the rod but with some difficulties; 6, no mistakes, symmetric movements.
Morris water maze
The Morris water maze task was used to assess spatial learning and memory. One week before treatment, aged rats were trained to find a submerged platform in a large (180-cm diameter) pool filled to within 20 cm of the upper edge with water maintained at 26 °C. The pool was divided into four compass quadrants (north, south, east, and west). Several visual stimuli were placed in each of the four quadrants. For the acquisition of spatial learning, each animal underwent a block of four trials per day for 7 days. Before the first trial, the rat was placed on the hidden platform for 30 s by the investigator. Each trial consisted of placing the rat in the water at one of the randomly selected four starting locations around the pool perimeter. Each rat was allowed a maximum of 60 s to find the hidden platform and remain on it for 30 s. If a rat failed to find the platform within 60 s, the rat was placed on the platform for 30 s by the investigator.
The time and distance required to find the hidden platform during these four acquisition trials were averaged (Tottori et al. 2002). The swim path was recorded by an image analysis system (VideoMot2, TSE, Bad Homburg, Germany) that computed path length and percentage of time spent in each of 4 quadrants. Functional assessment of spatial learning and memory was estimated every 2 weeks and in total for 16 weeks.
Histological examination
After 250 days of treatment, five animals/group were sacrificed for histological analysis of the liver, heart, spleen, and kidney. The animals were anesthetized with a mix of xylazine/ketamine, and serum was collected by cardiac puncture. Then the heart, spleen, kidney, and liver were surgically removed from both controls and experimental groups. All of these organs were dissected, and selected tissue samples from each organ were stored in 10% formaldehyde over 24 h. Subsequently, these samples were processed using a Leica tissue processor (Leica, Germany). The paraffin embedding was performed with a Leica tissue embedding system. After this step, serial 3-μm thick sections were cut using a Leica rotary microtome. Then these sections were transferred on glass slides and stained with hematoxylin and eosin.
Biochemical methods
RNA extraction and RNA quality control
After 250 days of treatment, the brains of five animals/group (see “Histological examination”) were analyzed by qRT-PCR. After the tissue was homogenized, total RNA was extracted from microdissected tissue using TRIzol reagent (Invitrogen Life Technologies, Karlsruhe, Germany). Genomic DNA was removed using the RNeasy Plus kit (Qiagen).
Quantitative real-time PCR
For quantitative real-time PCR (qPCR), we synthesized cDNA from individual samples of total RNA with the high-capacity cDNA reverse transcription kit (Applied Biosystems, USA). The qPCR was performed in 96-well 0.1-ml thin-wall PCR plates (Applied Biosystems) in the StepOnePlus System (Applied Biosystems). Each 20 μl reaction contained 10 μl iQ SYBR Green Master Mix (BioRad Laboratories, Hercules, CA, USA), 2 μl gene-specific forward and reverse primer mix, and 8 μl pre-diluted cDNA. No template controls contained nuclease-free water instead. The cycling conditions were 3 min 95 °C to activate iTaq DNA polymerase followed by 45 cycles with 30 s denaturation at 95 °C, 30 s annealing at 58 °C, and 30 s elongation at 72 °C. At the end of the amplification cycles, melting curves were used to validate PCR product specificity. All samples were amplified in triplicate. Data were analyzed using the ΔΔCt method (Livak and Schmittgen 2001). The expression levels of genes of interest were normalized to the average of expression level of the two housekeeping genes (hypoxanthine guanine phosphoribosyltransferase 1, HPRT1, and ribosomal protein 19, RPL 19) from the same sample. The relative expression for a gene of interest was defined as the ratio of expression of the gene to that of the housekeeping gene. The fold change for a gene of interest was defined as the ratio of the relative expression in the treated animals to that in the controls. Eurofins, Germany, provided all primers.
ELISA analysis of autophagy
Proteins were isolated simultaneously with the RNA isolation. Briefly, proteins in the lower phase left after RNA extraction were precipitated with isopropanol. After centrifugation at 12,000 g for 10 min and removal of the supernatant, the pellet was washed five times with 300 mM guanidine hydrochloride in 95% ethanol and twice in ice-cold acetone prior to centrifugation. The dried protein pellet was dissolved in RIPA buffer and protein concentration determined by the Bradford assay. Next, the levels of two proteins associated with autophagy were measure by ELISA. The test principle applied in this kit is sandwich enzyme immunoassay. The microtiter plate provided in this kit has been pre-coated with an antibody specific to microtubule-associated protein 1 light chain 3 alpha (MAP1LC3a) or lysosome-associated membrane protein 1 (LAMP1) (antibodies-online, Aachen, Germany). Standards or samples are then added to the appropriate microtiter plate wells followed by a biotin-conjugated antibody specific to MAP1LC3a or LAMP1. Next, avidin conjugated to horseradish peroxidase (HRP) is added to each microplate well and incubated. Next, TMB substrate solution is added, and the enzyme-substrate reaction is terminated by the addition of sulfuric acid, and the color change is measured spectrophotometrically at a wavelength of 450 nm. The concentration of MAP1LC3a and LAMP1 in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Bioavailability of spermidine
Serum from controls and treated animals was obtained by cardiac puncture, and spermidine concentration was determined using an immunoassay (BioCat GmbH, Heidelberg, Germany).
Statistical analysis
Behavior, body weight, and liquid intake
The main effects of treatment and time and interactions of the two factors were analyzed using two-way ANOVA (GraphPad Software, San Diego, CA, USA), with treatment as between-subjects variable and time as within-subjects variable. For quantitative data, the results were expressed as mean ± standard deviation (mean ± SD). The between-groups analysis was performed using post hoc tests (Bonferroni) for multiple comparisons. The level of significance was set at P < 0.05, using two-tailed test.
RT-PCR, ELISA, and spermidine bioavailability in serum
The effect of treatment has been done by unpaired t test, two-tailed. Histological comparisons were done using the Mann-Whitney test. Survival statistics was done using the Gehan-Breslow-Wilcoxon test.
Results
Treatment with spermidine did not increase the maximum life span in middle-aged male Sprague-Dawley rats
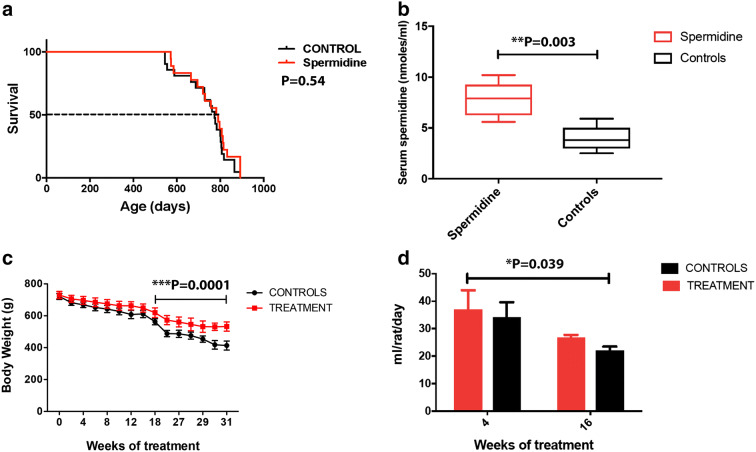
Treatment was initiated at the age of 18 months and continued for 350 days. The maximal survival was of 773 days for controls and 784 days for the treatment group. Neither the maximum nor the median life was significantly different between treatment and controls (Fig. 1a). Spermidine-fed animals displayed increased serum spermidine levels (7.8 nmol/ml serum) as compared to controls (3.9 nmol/ml serum), confirming its systemic bioavailability (Fig. 1b).
Treatment with spermidine slightly increased the median but not maximum life span in middle-aged male Sprague-Dawley rats. a Rat survival curves. Dashed lines depict median life spans. N = 41/44 (a, control/spermidine) male mice and N = 50/42 (b, control/spermidine) female mice. P value represents comparison with control group calculated using Breslow test. b Time course of the body weight during treatment. Note the significant difference of treatment on the body weight starting with week 18 of treatment. c The amount of liquid intake decreased significantly with increasing time, but there was no significant difference between the treated (spermidine) and control (water) groups. ***P = 0.0001 (ANOVA with post hoc Bonferroni); **P = 0.003 (unpaired t test, two-tailed); *P = 0.039 (ANOVA with post hoc Bonferroni); P = 0.054 (Gehan-Breslow-Wilcoxon test)
At the time the treatment was initiated, the body weights were roughly similar. However, the treatment led to a gradual increase in the body weight so that there was a significant weight differences between spermidine-supplemented and control rats starting at the 18th week of treatment (Fig. 1c). The amount of liquid intake decreased significantly with increasing time, but there was no significant difference between the treated and control groups (Fig. 1d).
Effects of long-term treatment with spermidine on behavior
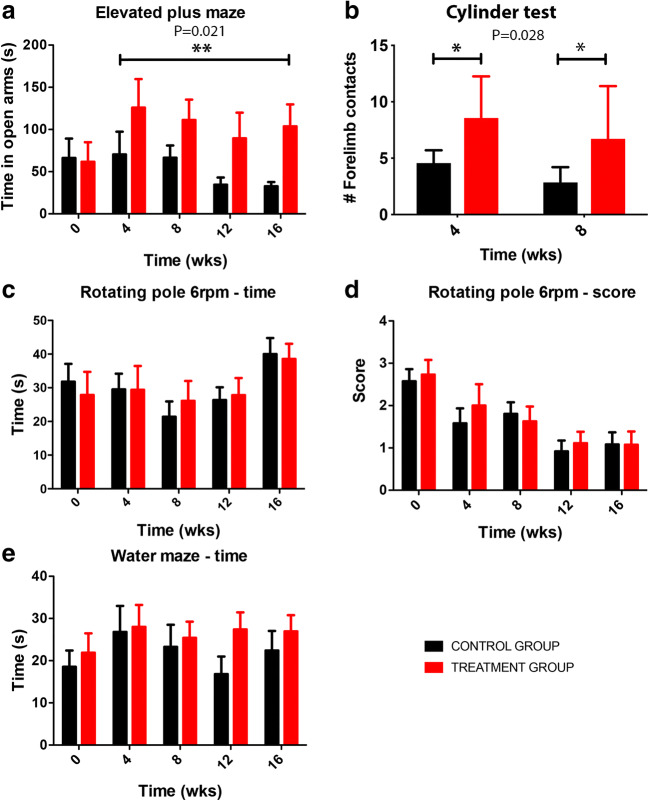
Behavior in the elevated plus maze reflects a conflict between the rodent’s preference for protected areas (closed arms) and their innate motivation to explore novel environments (open arms). Anxiety reduction is indicated in the plus maze by an increase in the proportion of time spent in the open arms. Accordingly, we found that the time spent in open arms was significantly larger in the treatment group starting at week 4 (Fig. 2a).
Effects of treatment with spermidine on behavior. Note a reduction in anxiety and an improvement in the exploratory behavior in the treatment group. There was no improvement in the beam walking (movement coordination) and water maze (working memory) tests. **P = 0.021 (ANOVA with post hoc Bonferroni); *P = 0.029 (ANOVA with post hoc Bonferroni)
The exploratory behavior of animals in a three-dimensional environment decreased with increasing age in both groups (Fig. 2b). However, the number of contacts with the forelimbs when exploring the wall of the cylinder in the upright position was significantly increased by spermidine administration both at 4 and 8 weeks of treatment (Fig. 2b).
On the beam walking test, both groups showed similar performance during the first 3 months of treatments. After that, performance began to deteriorate in both groups. In contrast to the time required to traverse the rotating beam, the score reflecting movement symmetry began to deteriorate progressively from the first week of testing and was not significantly modulated by treatment at both the 3- and 6-rpm speeds (Fig. 2c, d).
Over the training period of 7 days, rats learned to locate and climb onto the hidden platform and performance improved significantly during this time on the water maze test. However, after the treatment began, the performance did not improve in either group. On the contrary, the time needed to locate the platform slightly increased in both groups (Fig. 2e).
Spermidine treatment was associated with pathological changes in several organ
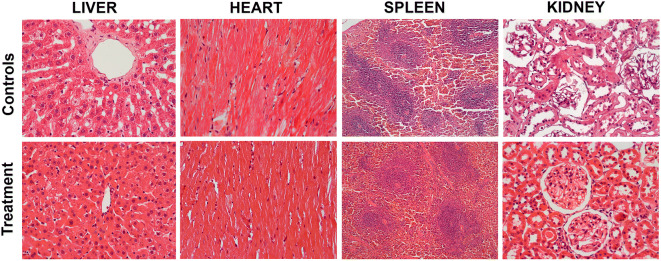
Histological evaluation of myocardium revealed focal degeneration of myocytes and focal necrosis and limited areas of fibrosis in both the control and experimental groups (Fig. 3). However, in the treated rats, lipomatosis was less evident, and there were fewer apoptotic myocytes. In the control group, we noted granulovacuolar degeneration of the epithelium of the kidney tubules, while this was less obvious in the experimental subjects. However, in both the aged control and experimental groups, the spleen showed normal architecture with focal mild accumulation of hemosiderin and giant multinucleated cells in the cords of Billroth (Fig. 3).
Effect of treatment with spermidine on histological features of the liver, heart, spleen, and kidney. Liver: (Controls): liver parenchyma with normal architecture; there is patchy focal inflammation and focal cellular degeneration surrounding the central portal vein. (Treatment): liver parenchyma with normal architecture; rare scattered inflammatory cells are seen in close proximity to the central portal vein. Heart: (Controls): myocardium showing normal architecture and some inflammatory cells admixed with some fibroblasts. (Treatment): myocardium showing normal architecture and less accumulation of fat. Spleen: (Controls): splenic parenchyma with normal architecture. (Treatment): splenic parenchyma with normal architecture. Kidney: (Control): renal parenchyma with focal glomerulosclerosis and changes suggestive of tubular atrophy. (Treatment): no evidence of glomerulosclerosis is seen. The most significant difference was observed in the liver (P = 0.029, Mann-Whitney test) and kidney (P = 0.036, Mann-Whitney test). Original magnification: 40x
Histopathological evaluation of the liver tissue in controls revealed peri-centrolobular, periportal, and medio-lobular patchy degeneration. Remarkably, these morphological changes were limited in the experimental group. Occasionally, we have also noted peri-centrolobular infiltration of lymphocytes in both groups (Fig. 3). However, chronic inflammation as suggested by the presence of the lymphocytes in the tissue was diminished in treated animals as compared with controls indicating a potential protective role of the administered spermidine on the liver. The histopathological changes are summarized in Table Table11.
Table 1
Histological evaluation of organs
| Pathological changes | Controls | Treatment |
|---|---|---|
| Heart | ||
| Low (+) | 7/11 | 8/9 |
| Medium (++) | 1/11 | 1/9 |
| High (+++) | 3/11 | 0/9 |
| Liver | ||
| Low (+) | 4/11 | 7/9 |
| Medium (++) | 4/11 | 2/9 |
| High (+++) | 3/11 | 0/9 |
| Kidney | ||
| Low (+) | 5/11 | 8/9 |
| Medium (++) | 6/11 | 1/9 |
| High (+++) | 0/11 | 0/9 |
| Spleen | ||
| Low (+) | 1/11 | 5/9 |
| Medium (++) | 3/11 | 3/9 |
| High (+++) | 7/11 | 1/9 |
Numerators indicate the number of rats with various
Degrees (low, medium, high) of pathology
Denominators indicate the total number of rats examined
Spermidine treatment attenuates neuroinflammation
It has been suggested that spermidine may have an anti-inflammatory effect by inhibiting proinflammatory cytokine synthesis in human mononuclear cells (Zhang et al. 1997) and suppressing LFA-1 expression on human lymphocytes (Soda et al. 2005). Indeed, chronic inflammation as suggested by the presence of the lymphocytes in the liver points out to less pathological changes in treated animals compared with the controls indicating a potential protective role of the administered factor.
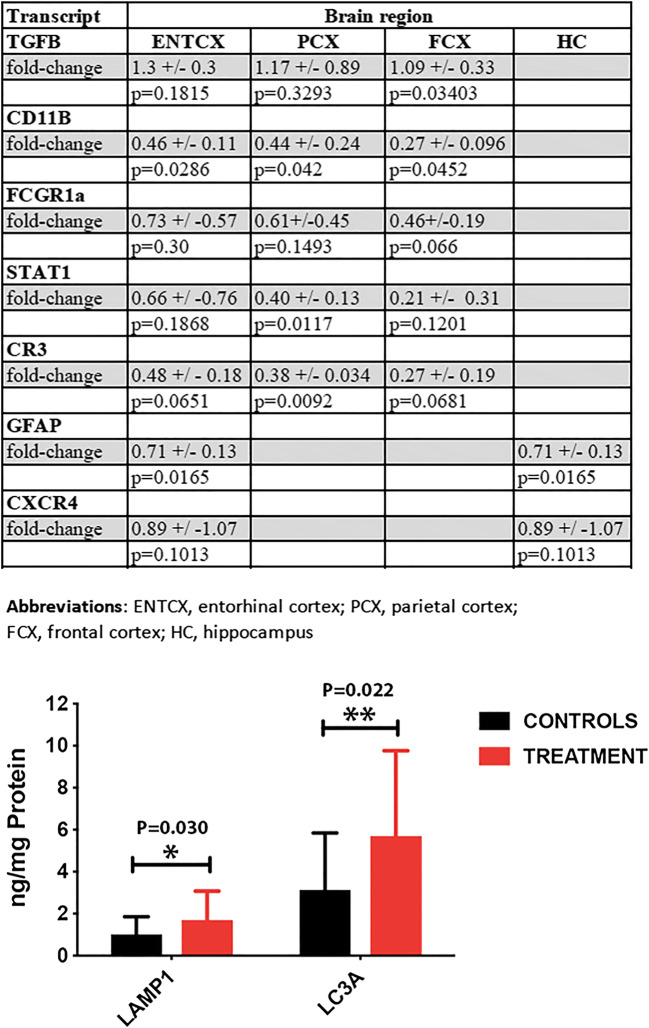
Further, we asked if long-term treatment with spermidine reduces gene expression for several inflammatory markers including CD11b, CR3, Tgfb, CXCR4, Fcgr1a, and Stat1 mRNAs. The expression of the astrocytic marker GFAP was also tested. Of these, we found, by RT-PCR, significant decreases in the expression of inflammatory and astrocytic marker in the transcripts coding for Tgfb, CD11b, Fcgr1, Stat1, CR3, and GFAP mRNAs in several cortical region and hippocampus of treated rats (Fig. 4, upper panel). CXCR4 coding for a chemokine receptor specific for stromal-derived-factor-1, also called Cxcl12, expression was not significantly changed by the treatment.
(Upper panel) Spermidine treatment attenuates neuroinflammation by reducing gene expression for several inflammatory markers including CD11b, CR3, Fcgr1a, Stat1, Tgfb, and GFAP mRNAs in several cortical regions and the hippocampus of the treated rats. (Lower panel) Long-term treatment with spermidine led to a small but significant increase in the levels of MAP1B-LC3a (P = 0.022, unpaired t test, two-tailed) and LAMP1 (P = 0.030, unpaired t test, two-tailed), two autophagic and endolysosomal organelle markers in neurons
Spermidine treatment enhances autophagy in the brain
It has been suggested that the mechanisms underlying the beneficial effects of spermidine on cardiac structure and function and life span extension is an increased autophagy (Madeo et al. 2018; Eisenberg et al. 2009, 2016). Indeed, long-term treatment with spermidine led to a small but significant increase in the levels of MAP1B-LC3a and LAMP1, two autophagic and endolysosomal organelle marker in neurons (Cheng et al. 2018; Wang et al. 2006); those levels are a good indicator of the autophagic flux (Fig. 4, lower panel).
Discussion
Living a longer, healthier life brings benefits to individuals, employers, and wider society that will be increasingly valuable in an aging population. Previous studies have shown that the polyamine spermidine had cardioprotective effects in mice and increased the maximum life span in C. elegans and extended both the maximum and median life span in mice (Eisenberg et al. 2016). A similar effect was achieved by food supplementation with probiotics that amplified gut microbiota polyamine synthesis or reduced midlife mortality by a polyamine-rich diet in short-lived mouse strains (Soda et al. 2009; Matsumoto et al. 2011).
Recently, a small population-based study has shown that nutrition rich in spermidine is linked to increased survival in humans. The survival advantage was driven by a reduced risk of death from all major causes (Kiechl et al. 2018). In our experiment, however, treatment with spermidine at 3 mM concentration in drinking water did not extend either the median or the maximum life span in middle-aged male Sprague-Dawley rats fueling the debate about the translatability of results across different species.
Let alone calorie restriction (Al-Regaiey 2016), life span extension in higher organisms has proven to be more difficult to achieve using simple drugs. Along this line, a ketogenic diet increased median life span and survival and slowed age-related decline in physiological function in mice compared to controls by increasing protein acetylation levels and regulating mTORC1 signaling (Roberts et al. 2017). However, a previous study reported that lifelong feeding with a ketogenic diet did not alter longevity in C57BL/6 mice suggesting that feeding strategies and husbandry issues may play a role in determining the influence of the ketogenic diet on life span (Douris et al. 2015). Similarly, rapamycin given to mice seems to prevent age-dependent decline in spontaneous activity and retard age-related pathology, including degenerative changes in tendon elasticity, liver, heart, and endometrium the liver and heart. However, rapamycin treatment also led to a pronounced testicular tubular degeneration in mice confirming that spermatogenesis and fertility are impaired in men receiving rapamycin following therapeutic transplantation (Wilkinson et al. 2012).
The polyamines spermidine and spermine have pleiotropic effects on cell physiology, some of which are thought to influence animal behavior (Guerra et al. 2016). Indeed, long-term treatment of middle-aged rats with spermidine led to a reduction in anxiety, as indicated by an increase in the proportion of time spent in the open arms of the plus maze and by an improvement in exploratory performance in the cylinder test. However, the treatment did not improve performance on spatial memory or bilateral sensorimotor coordination (rotating beam) tasks. Given the beneficial effects of increased autophagy and decreased neuroinflammation on the aging process (Madeo et al. 2018; López-Otín et al. 2016; Madeo et al. 2015; Sandu et al. 2015), we speculate that manipulating brain autophagy and neuroinflammation with spermidine may also have beneficial effects on behavior including a reduction in anxiety and an increase in curiosity as assessed by exploratory behavior in animal models of aging.
Long-term treatment of mice fed with food containing spermine and spermidine or more recently administration of the polyamines spermine and spermidine in drinking water extended the median life span of young mice as compared to that of controls by enhancing cardiac autophagy and a significant decrease of several plasma proteins including chitinase-3-like protein 1 (CHI3L1) — associated with pathogenic processes related to inflammation (Zhang et al. 1997; Soda et al. 2005, 2009; Eisenberg et al. 2016). However, behavior was not investigated in these studies.
Aging leads to a progressive decline in immune function which is associated with an increased frequency of infections and chronic diseases. In particular, aged men experience a significant decrease in the levels of circulating testosterone and dehydroepiandrosterone (DHEA), which have been linked to depression, cardiovascular diseases, and sarcopenia. Interestingly, short-term physiological androgen supplementation in aged male rhesus macaques led to improved immune senescence by stabilizing the frequency of naïve and memory T cells decreasing the levels of inflammatory cytokines in supplemented aged males compared to the aged controls (Rais et al. 2017).
The aging brain is characterized by a proinflammatory state mainly due an increased numbers of activated and primed microglia and increased steady state levels of inflammatory cytokines. As a result, an exaggerated inflammatory responses may lead to the development of cognitive deficits, impaired synaptic plasticity, and accelerated neurodegeneration (Patterson 2015). Although cognitive decline is observed in the normal aging monkey, neurons are seemingly not lost with increasing age. Instead, frontal white matter is lost as myelin degenerates and both correlate with age-related cognitive decline. One underlying mechanism of cognitive decline in aging monkey could be an age-related increase in myelin damage. Indeed, using unbiased stereology to quantify the density of activated microglia and phagocytic microglia, it was shown that microglia become activated to a phagocytic phenotype with increasing age, most likely in response to accumulating myelin pathology in the white matter (Shobin et al. 2017).
Spermidine can prevent memory loss in aging model organisms (Gupta et al. 2013, 2016). Promotion of cognitive and brain health in older individuals with cognitive decline is one of the most crucial public health issues. A small clinical trial tested whether spermidine supplementation has a positive impact on memory performance in patients with cognitive decline and progression to dementia due to Alzheimer’s disease. Indeed, memory performance was moderately enhanced in the spermidine group compared with placebo at the end of intervention (Wirth et al. 2018).
The age-related loss of muscle mass and function in older adults may be the blunted response of skeletal muscle protein synthesis after dietary protein feeding which can be attributed to with the anabolic resistance due to elevated levels of oxidative stress and inflammation (Balage et al. 2010). Decreasing oxidative stress and inflammation through antioxidant or ibuprofen treatment has been shown to restore the acute anabolic effects of leucine-stimulated mixed muscle protein synthesis in animals (Rieu et al. 2009).
In a recent study, it was shown that 6 weeks of dietary supplementation with conjugated linoleic acid (CLA) and Protandim, which have been shown to activate nuclear factor erythroid-derived 2-like 2 (Nrf2), a transcription factor that regulates the expression of the endogenous antioxidant network and anti-inflammatory pathways, may enhance proteostatic mechanisms of skeletal muscle contractile proteins in older adults and the age-related loss of muscle mass and function (Konopka et al. 2017). In this context, our finding that the treatment of the middle-aged rats with spermidine led to an increase in the body weight offers a promising alternative to prevent body weight loss with increasing age by dietary supplementation with spermidine.
In our study, treatment with spermidine alone enhanced autophagy in the brain and led to a diminished expression of the inflammatory markers, Tgfb, CD11b, Fcgr1, Stat1, CR3, and GFAP mRNAs in several cortical region and hippocampus of the treated rats suggesting that one beneficial effect of the long-term treatment with spermidine is an attenuated proinflammatory state in the aged brain.
Long-term treatment with spermidine in mice had a protective effect both on the heart and the kidney (Eisenberg et al. 2009). Senescence is associated with significant histological changes in the kidney. Elderly subjects develop nephrosclerosis which is associated with focal and global glomerulosclerosis, as well as interstitial fibrosis and tubular atrophy (Denic et al. 2016; Anderson and Brenner 1986; Bolton and Sturgill 1980). In addition, histopathological evaluation of senescent kidney reveals significant fibro-intimal thickening which indicates arteriosclerosis (Denic et al. 2016). This has a major role promoting an ischemic injury which leads to pericapsular fibrosis and alteration in the basement membrane functionality, while the Bowman’s capsule fills with a proteinaceous hyaline material (Denic et al. 2016). In our experiments, focal glomerulosclerosis is present in both control and experiments groups, although it was more obvious in controls.
Spermidine given in the drinking water exerted cardioprotective effects, reducing cardiac hypertrophy and preserving diastolic function in old mice by increased cardiac autophagy, mitophagy, and mitochondrial respiration. The spermidine treatment also improved the mechano-elastical properties of cardiomyocytes in vivo (Eisenberg et al. 2016). Cardiac senescence and myocardial fibrosis have been previously described in the literature, but the underlying mechanism is poorly understood. It was reported that blocking p53 via siRNA in cardiac fibroblasts will significantly decrease senescence associate hypoxic injury, while an increased level of endogenous p53 increases ischemia associated with senescence. This suggests that p53 could be a potential therapeutic target in this context (Zhu et al. 2013). Although molecular mechanisms of cardiac senescence are incompletely elucidated, evaluation of myocardial fibrosis is of paramount importance for histological assessment of cardiac senescence. In our study, we confirm the cardioprotective effects of spermidine which had a beneficial effect on heart morphology by diminishing the accumulation of fat and the number of dying myocytes.
With regard to liver senescence, Ogrodnik et al. (2017) have reported that it is associated with nonalcoholic fatty liver disease (NAFLD) characterized by an accumulation of lipids suggesting steatosis. The authors have described in a murine experimental model that a blockage of p16INK4a-positive senescent cells in genetically modified subjects (INK-ATTAC mice) decreases liver steatosis suggesting a potential therapeutic target (Ogrodnik et al. 2017).
In this context, previous studies have shown that young male mice fed orally high polyamine chow for 64 weeks showed lower incidence of glomerulosclerosis and increased expression of senescence marker protein-30 (SMP-30) in both kidney and liver compared to those fed the low polyamine chow. SMP-30 has been shown to protect organs from oxidative stress during aging, and its tissue levels decrease with aging (Fujita et al. 1992; Sato et al. 2009). Thus, the preservation of SMP-30 staining indicates that the progression of age-associated pathologies is attenuated in mice fed the high polyamine chow (Soda et al. 2009). In our study, the treatment with spermidine did not alter either fibrosis in the myocardium or the accumulation of hemosiderin and giant multinucleated cells in the hepatic cords of Billroth. However, the granulovacuolar degeneration of the epithelium of the kidney tubules was attenuated in the experimental subjects and had a beneficial effect on peri-centrolobular, periportal, and medio-lobular hepatic patchy degeneration in spermidine-treated animals.
Conclusion
Our results suggest that long-term treatment with spermidine increases health span of middle-aged rats by attenuating neuroinflammation, anxiety, and the exploratory behavior. Mice and rats, let alone C. elegans and D. melanogaster, differ genetically, metabolically, anatomically, and behaviorally. Hence, our finding that they also differ in response to the putative longevity-extending agent spermidine may not come as a surprise. However, the results add fuel to the debate about the translatability of the results such treatments among species and in particular to humans.
Authors’ contributions
MF, IU, and AO performed the experiments. CM assessed the pathology; EP and DMH contributed to the design of the experiments and to the writing of the manuscript. APW designed the experiments and wrote the manuscript.
Funding information
This work was supported by the EU Framework Programme for Research and Innovation, Horizont 2020, project number 667302 to APW, and UEFISCDI, project numbers PN-III-P4-ID-PCE-2016-0340 to DH, PN-III-P2-2.1-PED-2016-1013, and PN-III-P4-ID-PCE-2016-0215 to APW.
Compliance with ethical standards
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Andrei Olaru and Ion Udristoiu contributed equally to this work.
References
- Abolaji AO, Adedara AO, Adie MA, Vicente-Crespo M, Farombi EO. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem Biophys Res Commun. 2018;503:1042–1048. doi: 10.1016/j.bbrc.2018.06.114. [PubMed] [CrossRef] [Google Scholar]
- Al-Regaiey KA. The effects of calorie restriction on aging: a brief review. Eur Rev Med Pharmacol Sci. 2016;20:2468–2473. [PubMed] [Google Scholar]
- Anderson S, Brenner BM. Effects of aging on the renal glomerulus. Am J Med. 1986;80:435–442. doi: 10.1016/0002-9343(86)90718-7. [PubMed] [CrossRef] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Balage M, Averous J, Rémond D, Bos C, Pujos-Guillot E, Papet I, Mosoni L, Combaret L, Dardevet D. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2010;21:325–331. doi: 10.1016/j.jnutbio.2009.01.005. [PubMed] [CrossRef] [Google Scholar]
- Bolton WK, Sturgill BC. Spontaneous glomerular sclerosis in aging Sprague-Dawley rats. II. Ultrastructural studies. Am J Pathol. 1980;98:339–356. [PMC free article] [PubMed] [Google Scholar]
- Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Popa-Wagner A. Environmental enrichment improves functional and neuropathological indices following stroke in young and aged rats. Restor Neurol Neurosci. 2007;25:1–18. [PubMed] [Google Scholar]
- Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, Partridge L. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr Biol. 2018;28:1714–1724.e4. doi: 10.1016/j.cub.2018.04.015. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cheng XT, Xie YX, Zhou B, Huang N, Farfel-Becker T, Sheng ZH. Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. J Cell Biol. 2018;217:3127–3139. doi: 10.1083/jcb.201711083. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Collins JJ, Evason K, Pickett CL, Schneider DL, Kornfeld K. The anticonvulsant ethosuximide disrupts sensory function to extend C. elegans lifespan. PLoS Genet. 2008;4:e1000230. doi: 10.1371/journal.pgen.1000230. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Danilov A, Shaposhnikov M, Shevchenko O, Zemskaya N, Zhavoronkov A, Moskalev A. Influence of non-steroidal anti-inflammatory drugs on Drosophila melanogaster longevity. Oncotarget. 2015;6:19428–19444. doi: 10.18632/oncotarget.5118. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Douris N, Melman T, Pecherer JM, Pissios P, Flier JS, Cantley LC, Locasale JW, Maratos-Flier E. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim Biophys Acta. 2015;1852:2056–2065. doi: 10.1016/j.bbadis.2015.07.009. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [PubMed] [CrossRef] [Google Scholar]
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Büttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Mühlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Fujita T, Fukase M, Baba H, Yamaguchi T, Takata S, Fujimi T, Nishikawa M, Nakamoto C. New actions of parathyroid hormone through its degradation. J Endocrinol Investig. 1992;15:121–127. doi: 10.1007/BF03348676. [PubMed] [CrossRef] [Google Scholar]
- Guerra GP, Rubin MA, Mello CF. Modulation of learning and memory by natural polyamines. Pharmacol Res. 2016;112:99–118. doi: 10.1016/j.phrs.2016.03.023. [PubMed] [CrossRef] [Google Scholar]
- Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KS, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16:1453–1460. doi: 10.1038/nn.3512. [PubMed] [CrossRef] [Google Scholar]
- Gupta VK, Pech U, Bhukel A, Fulterer A, Ender A, Mauermann SF, et al. Spermidine suppresses age-associated memory impairment by preventing adverse increase of presynaptic active zone size and release. PLoS Biol. 2016;14(9):e1002563. 10.1371/journal.pbio.1002563. [PMC free article] [PubMed]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hussain SS, Ali M, Ahmad M, Siddique KHM. Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv. 2011;29:300–311. doi: 10.1016/j.biotechadv.2011.01.003. [PubMed] [CrossRef] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [PubMed] [CrossRef] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, Werner, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108:371–380. doi: 10.1093/ajcn/nqy102. [PubMed] [CrossRef] [Google Scholar]
- Konopka AR, Laurin JL, Musci RV, Wolff CA, Reid JJ, Biela LM, Zhang Q, Peelor FF, 3rd, Melby CL, Hamilton KL, Miller BF. Influence of Nrf2 activators on subcellular skeletal muscle protein and DNA synthesis rates after 6 weeks of milk protein feeding in older adults. Geroscience. 2017;39:175–186. doi: 10.1007/s11357-017-9968-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kumar S, Dietrich N, Kornfeld K. Angiotensin converting enzyme (ACE) inhibitor extends Caenorhabditis elegans life span. PLoS Genet. 2016;12:e1005866. doi: 10.1371/journal.pgen.1005866. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lashmanova E, Proshkina E, Zhikrivetskaya S, Shevchenko O, Marusich E, Leonov S, Melerzanov A, Zhavoronkov A, Moskalev A. Fucoxanthin increases lifespan of Drosophila melanogaster and Caenorhabditis elegans. Pharmacol Res. 2015;100:228–241. doi: 10.1016/j.phrs.2015.08.009. [PubMed] [CrossRef] [Google Scholar]
- Lewis KN, Rubinstein ND, Buffenstein R. A window into extreme longevity; the circulating metabolomic signature of the naked mole-rat, a mammal that shows negligible senescence. Geroscience. 2018;40:105–121. doi: 10.1007/s11357-018-0014-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [PubMed] [CrossRef] [Google Scholar]
- López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [PubMed] [CrossRef] [Google Scholar]
- Madeo F, Zimmermann A, Maiuri MC, Kroemer G. Essential role for autophagy in life span extension. J Clin Invest. 2015;125:85–93. doi: 10.1172/JCI73946. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359(6374):eaan2788. doi: 10.1126/science.aan2788. [PubMed] [CrossRef] [Google Scholar]
- Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y. Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on Upregulation of gut bacterial polyamine production. PLoS One. 2011;6:e23652. doi: 10.1371/journal.pone.0023652. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de CR FE, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Niraula P, Ghimire S, Lee H, Kim MS. Ilex paraguariensis extends lifespan and increases an ability to resist environmental stresses in Drosophila. Rejuvenation Res. 2018;21. 10.1089/rej.2017.2023. [PubMed]
- Nishimura K, Shiina R, Kashiwagi K, Iagarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139:81–90. doi: 10.1093/jb/mvj003. [PubMed] [CrossRef] [Google Scholar]
- Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology. 2015;96:11–18. doi: 10.1016/j.neuropharm.2014.12.020. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rais M, Wilson RM, Urbanski HF, Messaoudi I. Androgen supplementation improves some but not all aspects of immune senescence in aged male macaques. Geroscience. 2017;39:373–384. doi: 10.1007/s11357-017-9979-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, McMackin MZ, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA. A Ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26:539–546.e5. doi: 10.1016/j.cmet.2017.08.005. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [PubMed] [CrossRef] [Google Scholar]
- Sandu RE, Buga AM, Uzoni A, Petcu EB, Popa-Wagner A. Neuroinflammation and comorbidities are frequently ignored factors in CNS pathology. Neural Regeneration Research. 2015;10:1349–1355. doi: 10.4103/1673-5374.165208. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sato T, Wu X, Shimogaito N, Takino J, Yamagishi S, Takeuchi M. Effects of high-AGE beverage on RAGE and VEGF expressions in the liver and kidneys. Eur J Nutr. 2009;48:6–11. doi: 10.1007/s00394-008-0753-4. [PubMed] [CrossRef] [Google Scholar]
- Shobin E, Bowley MP, Estrada LI, Heyworth NC, Orczykowski ME, Eldridge SA, Calderazzo SM, Mortazavi F, Moore TL, Rosene DL. Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience. 2017;39:199–220. doi: 10.1007/s11357-017-9965-y. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Soda K, Kano Y, Nakamura T, Kasono K, Kawakami M, Konishi F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J Immunol. 2005;175:237–245. doi: 10.4049/jimmunol.175.1.237. [PubMed] [CrossRef] [Google Scholar]
- Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44:727–732. doi: 10.1016/j.exger.2009.08.013. [PubMed] [CrossRef] [Google Scholar]
- Tottori K, Nakai M, Uwahodo Y, Miwa T, Yamada S, Oshiro Y, Kikuchi T, Altar CA. Attenuation of scopolamineinduced and age-associated memory impairments by the sigma and 5-hydroxytryptamine(1A) receptor agonist OPC-14523 (1-10.1007/s11357-019-00152-5 [3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2[1H]-quino linone monomethanesulfonate). J Pharmacol Exp Ther. 2002;301(1):249–257. [PubMed]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [PubMed] [CrossRef] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8868. doi: 10.1523/JNEUROSCI.2261-06.2006. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wirth M, Benson G, Schwarz C, Köbe T, Grittner U, Schmitz D, Sigrist SJ, Bohlken J, Stekovic S, Madeo F, Flöel A. The effect of spermidine on memory performance in older adults at risk for dementia: a randomized controlled trial. Cortex. 2018;109:181–188. doi: 10.1016/j.cortex.2018.09.014. [PubMed] [CrossRef] [Google Scholar]
- Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhu F, Li Y, Zhang J, Piao C, Liu T, Li HH, Du J. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One. 2013;8:e74535. doi: 10.1371/journal.pone.0074535. [PMC free article] [PubMed] [CrossRef] [Google Scholar]