- Journal List
- GeroScience
- v.43(2); 2021 Apr
- PMC8110650

C60 in olive oil causes light-dependent toxicity and does not extend lifespan in mice
 6 and Kelsey J. Moody1
6 and Kelsey J. Moody1Kristopher J. Grohn
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
2Department of Chemistry, College of Environmental Science and Forestry, State University of New York, Syracuse, NY 13210 USA
Brandon S. Moyer
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Danique C. Wortel
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Cheyanne M. Fisher
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Ellie Lumen
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
3Betterhumans Inc., Gainesville, FL USA
Anthony H. Bianchi
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Kathleen Kelly
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Paul S. Campbell
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Douglas E. Hagrman
4Department of Chemistry and Physical Sciences, State University of New York, Onondaga Community College, Syracuse, NY 13215 USA
Roger G. Bagg
5BioSenex, Ltd., Lyndhurst, 1 Cranmer Street, Nottingham, Nottinghamshire, NG10 1NJ UK
Aaron J. Wolfe
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
Andrea Basso
6Advanced Technology Center for Aging Research, IRCCS INRCA, via Birarelli 8, 60121 Ancona, Italy
Cristina Nicoletti
6Advanced Technology Center for Aging Research, IRCCS INRCA, via Birarelli 8, 60121 Ancona, Italy
Giovanni Lai
6Advanced Technology Center for Aging Research, IRCCS INRCA, via Birarelli 8, 60121 Ancona, Italy
Mauro Provinciali
6Advanced Technology Center for Aging Research, IRCCS INRCA, via Birarelli 8, 60121 Ancona, Italy
Marco Malavolta
6Advanced Technology Center for Aging Research, IRCCS INRCA, via Birarelli 8, 60121 Ancona, Italy
Kelsey J. Moody
1Ichor Therapeutics, Inc., 2521 US Route 11, LaFayette, NY 13084 USA
 Corresponding author.
Corresponding author.Associated Data
Abstract
C60 is a potent antioxidant that has been reported to substantially extend the lifespan of rodents when formulated in olive oil (C60-OO) or extra virgin olive oil (C60-EVOO). Despite there being no regulated form of C60-OO, people have begun obtaining it from online sources and dosing it to themselves or their pets, presumably with the assumption of safety and efficacy. In this study, we obtain C60-OO from a sample of online vendors, and find marked discrepancies in appearance, impurity profile, concentration, and activity relative to pristine C60-OO formulated in-house. We additionally find that pristine C60-OO causes no acute toxicity in a rodent model but does form toxic species that can cause significant morbidity and mortality in mice in under 2 weeks when exposed to light levels consistent with ambient light. Intraperitoneal injections of C60-OO did not affect the lifespan of CB6F1 female mice. Finally, we conduct a lifespan and health span study in males and females C57BL/6 J mice comparing oral treatment with pristine C60-EVOO and EVOO alone versus untreated controls. We failed to observe significant lifespan and health span benefits of C60-EVOO or EVOO supplementation compared to untreated controls, both starting the treatment in adult or old age. Our results call into question the biological benefit of C60-OO in aging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-020-00292-z.
Introduction
Since its discovery in 1985, C60 fullerene has captured the attention of chemists and biologists alike because of its unique chemistry and potential biological applications. Its relatively large size (~ 0.7 nm), hollow sphere architecture, extensive electrical conjugation, electrophilicity, symmetry, low toxicity, and ability to accumulate in biological tissues all present unique opportunities to apply C60 in a wide array of applications from materials science to medicine. For example, functionalized C60 derivatives have been investigated as anti-cancer photosensitizers in photodynamic therapy [1], anti-viral molecules as inhibitors of HIV protease [2], drug-delivery vectors for peptides [3], genes and other large molecules [4, 5], and diagnostic imaging agents as metal conjugates [6], among other applications.
C60 has also gained considerable attention for its potential as an antioxidant and ROS scavenger [7]. C60 and C60 derivatives have been shown to scavenge a number of free radicals, including superoxide and hydroxyl radicals [8–10], in addition to singlet oxygen and the stable 2,2-diphenyl-1-picryhydrazyl radical [10]. This combined with observations that various C60 derivatives can localize to the mitochondrial membrane and that C60 can affect mitochondrial function in vitro [11–13] has caused some to hypothesize that C60 might be able to localize to the mitochondrial membrane in vivo and detoxify mitochondrial ROS. According to the hypothesis, this should prevent cellular damage and mitigate the long-term toxicity of mitochondrial ROS, perhaps even extending healthy lifespan should the mitochondrial or free radical theories of aging prove to be significant contributors to human longevity.
One of the more striking results in C60 literature was the observation that a small cohort of rats that were dosed with C60 dissolved in olive oil (C60-OO) from youth had extended mean lifespans (90% increase) relative to rats who were only dosed with olive oil (OO) or with nothing at all [14]. This is the largest life-extension effect on rats from a chemical compound seen to date, and it is the only study of C60 on lifespan. A precedent study provided evidence of harmful effects of C60 on mouse embryos in vitro and in vivo [15] while other studies have been focused on the protective properties of C60 in specific models of injury [16, 17]. Another study on rodent’s lifespan has been performed with a derivative of fullerene, carboxyfullerenes, which display important physical and chemical differences from C60. Mice treated with the carboxyfullerene lived 11% longer than control mice with improved cognitive performance [18]. Due to the potential medical benefits of a small molecule capable of extending lifespan and the discrepancy in reported results, the independent confirmation of the effects of C60 on lifespan is extremely valuable. Indeed, the previous lifespan results are so compelling that some communities have begun to treat themselves and their domestic pets with C60-OO obtained from online vendors, even though the drug product and formulation have not been assessed for toxicity by any regulatory agency [14]. The lack of formulation and storage quality control in these products due to their unregulated nature raises concerns relevant to the public health. C60 has been found to degrade under UV-irradiation in organic solvents, producing reddish brown deposits that contain aldehydes and other oxidation products [19]. Fullerene polymerization and aggregation have also been reported after light exposure, suggesting that multiple physical transformations could occur with C60 in solution [20–22]. Given that C60 is given to photooxidation, commercially purchased C60-OO could contain products that are potentially toxic.
In this study, we obtain C60-OO from a sample of popular online vendors and subject them to basic concentration and activity assays and find great variability. We then subject pristine C60-OO to a photodegradation test and demonstrate the disappearance of C60 and the appearance of toxic species in the formulation capable of causing death in mice. We also perform a lifespan study using intraperitoneal injection of pristine C60-OO on aged CB6F1 mice and do not find a significant lifespan benefit compared to OO alone. Finally, we performed a lifespan and health span study with oral administration of C60-EVOO or EVOO alone in C57BL/6 J mice and do not find a significant lifespan and health span effect compared to untreated controls. These results should raise questions regarding the efficacy of C60-OO as a supplement for extending lifespan, and of the potential toxicity of preparations caused by environmental stress.
Methods
Reagents
C60 powder was purchased from SES Research (Huston, TX). Pristine C60-OO samples were prepared in house by mixing 5 mg of C60 powder per milliliter of olive oil and stirring the solution for 2 weeks in the dark. C60-OO mixtures were then filtered through a 0.22-μm PTFE filter to remove any remaining solid C60. Commercial C60-OO was ordered from online sources and stored at room temperature in the dark. Fluorescent bulbs were sourced from Hydrofarm, Inc. (LKIT150). All other chemicals were purchased from Millipore Sigma (Burlington, MA). All data were analyzed using GraphPad Prism 8 (San Diego, CA) using the indicated statistical tests.
Husbandry
Animals for studies involving intraperitoneal injections (toxicity study in C57BL6/J and lifespan study in CB6F1 mice): C57BL6/J mice (5 groups; N = 4 per group) for the toxicity study were obtained from the Jackson Laboratory (stock #000664). Aged CB6F1 mice for the lifespan study (2 groups; N = 11 per group) were obtained from Betterhumans Inc. (Gainesville, FL). All mice were maintained in a specific pathogen free facility at Ichor Therapeutics, Inc (Lafayette, NY) with a 12-h light/dark schedule. Temperature was maintained between 20 and 22 °C and humidity was maintained between 30 and 70%. Standard mouse chow and chlorinated RO water were provided ad libitum. Animals were monitored daily. In the lifespan study, weights and food consumption were monitored daily.
Animals for the lifespan and health span study with oral administration of C60: C57BL/6 J (N = 66) mice were originally obtained from the Jackson Laboratory and bred in the animal facility of INRCA (Ancona, Italy). The mice were kept under conventional conditions at 23 ± 2 °C, ambient temperature, and 60 ± 15% relative humidity, with minimum ventilation rate of 10 times/h. Artificial light was allowed to follow cycles simulating natural seasonal light cycles. Animals were housed 3–4 per cage (polycarbonate, open on the top and covered with steel wire lid, 26.7 × 20.7 × 14.0 cm deep) and fed with conventional chow (Global diet 2018, Harlan, Italy) and tap water ad libitum.
C60 HPLC conditions
HPLC separation and analyses were performed using a PerkinElmer Flexar LC system with an attached PerkinElmer Flexar UV/Vis detector. Data acquisition was performed using Chromera software from PerkinElmer. The system was equipped with a Waters Atlantis dC18 column, 4.6 mm × 150 mm, 3 μm particle size. Separations were performed at ambient temperature with a flow rate of 0.5 mL/min using a mobile phase composed of toluene and acetonitrile (70/30, v/v). Spectra were recorded at 330 nm using a 10 μL injection volume. Samples of C60-OO were diluted 1:10 in toluene and passed through a 0.22-μm nylon filter prior to analysis. Peak identity and standard curves were generated using a standard solution of C60 in toluene.
Antioxidant capacity assay
C60-OO sourced from 4 online vendors were compared to freshly made samples which were prepared and stored in the dark. Samples were diluted using unmodified olive oil to a concentration of 0.5 mg/mL. Oxidation was performed by adding 3.2 mL of an oxidation solution composed of 30% hydrogen peroxide, acetic acid, and sulfuric acid (84/14/2, v/v) to 7.8 mL of C60-OO. Samples were stirred for 2 hours at room temperature, and then washed using 3 volumes of deionized water. 1.0 mL aliquots of the oxidized oil were then treated with 3.00 mL of 8.3% (w/v) 4-(4-nitrobenzyl)pyridine in methyl ethyl ketone. Samples were incubated at 65 °C for 3 h, then cooled to room temperature. Samples were then mixed with 1 mL of 50% triethylamine in acetone to initiate color development and were assayed immediately at 565 nm in a UV-Vis spectrophotometer.
Light exposure toxicity
Freshly prepared C60-OO was irradiated with the light of a full daylight spectrum 32 W fluorescent bulb (380–700 nm range) for 8 days. Samples were exposed to at least 70,000 Lux light intensity as measured by an Extech-LT40 light meter. Aliquots of C60-OO were drawn on 0, 2, 4, 6, and 8 days of light exposure and were assayed for C60 content by HPLC then stored in the dark. Samples of C60-OO under varying degrees of light exposure and a control sample of light irradiated olive oil were then sterile filtered and delivered via single IP injections to age matched male C57BL6/J mice (n = 4) at 4 mg/kg (2.85 mL/kg, ~ 57 μL/20 gram average weight) dosages of the original C60 content. Animals were monitored for their survival rates over 14 days.
Aged CB6F1 mice lifespan study
Female CB6F1 mice between 100 and 109 weeks of age were randomly distributed into two groups of 11 mice. The groups received 1.7-mg/kg dosages of C60-OO or an equivalent volume of unmodified olive oil (OO) via intraperitoneal injection. Mice were injected under a dosage schedule similar to that of previous performed studies by Baati et al [14]. Briefly, the mice received injections daily for the first week, then weekly for 7 weeks, then fortnightly for twenty more weeks.
Replication of lifespan and health span study in C57BL6J mice
For this study, we used a total of 66 C57BL/6 J mice (34 males and 32 females). For the treatments with EVOO and C60-EVOO, we used a young/adult group (18 C57BL/6 J mice) aged 4.7–5.1 months (5 months in average, 9 males and 9 females) and an old group (20 C57BL/6 J mice) aged 18.8 months (10 males and 10 females). Mice were adapted to the phenotype manipulation for 5 months before starting the treatment. After this period, the mice were supplemented with EVOO or C60-EVOO, starting from 9 months of age for the younger group (named EVOO_Y and C60 + EVOO_Y) and from 23 months of age for the older group (named EVOO_O and C60 + EVOO_O). As control group, we used 28 mice aged 16 months (13 females and 15 males) that served as untreated controls for another study performed in the same period. Comparison of lifespan and health span among control and supplemented groups is reported for all mice still alive from the age of 21 months. The experimental design, the period during which the treatment started, and the number of mice surviving along the study are described in Supplementary table 2.
Treatment
Eighty milligrams of fullerene C60 (SES Research, Huston TX, USA) was dissolved in 10 ml of extra virgin olive oil (EVOO) by stirring for 2 weeks at ambient temperature in the dark. For this purpose, commercial, high-quality EVOO from Central Italy was used. The resulting mixture was centrifuged at 5.000g for 1 h and the supernatant was filtered through a Millipore filter with 0.25 μm porosity. As the solubility of C60 varies from 0.6 to 1.2 mg/ml, according to the olive-oil composition and its moisture content, we confirmed that the final solution was at the concentration of 0.8 mg/ml by UV-Visible spectroscopy (absorbance of toluene diluted solutions at 330 nm).
Mice assigned to the treatment group were administered daily, for a week, 150 μl of the C60-oil solution, corresponding approximately to a mean dose of 4 mg/kg*bw of C60-OO. Control mice were administered with the same amount of EVOO. Administration of C60 and EVOO was performed by dispensing the solution with a pipette in the mouth of the mice. All mice, with no noted exception, voluntarily consumed all the dose. Administration of C60-OO was performed daily for the first week of the study, then weekly for 1 month, then fortnightly for 7 months.
Health-span assessment
Non-invasive mouse phenotyping included monthly measurements of body weight (BW, g), ventral surface temperature (ST), grip strength, and locomotor activity. Mice were adapted to the phenotype manipulation for 5 months before starting the treatment.
Grip strength was measured using the gripped weights method [23] with a slight adaptation to better discriminate changes of strength in old mice [24]. The resulting score (strength score, SS) was used in the analysis.
Locomotor activity was conducted by a 5-min open field test on a white wood-chamber (72 × 72 × 30 cm) surmounted by a Xiaomi Yi Camera 16MP 1080 P 60FPS (YI Technology) controlled via WI-Fi by a Smartphone. At the conclusion of all testing for the day, as well as before changes from an experimental group to another, the arena was sanitized with Virkon followed by 70% ETOH to remove any Virkon residue. Videos were collected in a microSD disk and the tracking was performed offline with Biobserve Viewer3 (Biobserve GmbH, Germany). The final variables that were included in the analysis were those recently used for a frailty screening: Track Length (cm), Activity (% the mice run at speed above 0.45 cm/s), and the highest speed interval that the mouse traveled for at least 3 s [25]. For this purpose, the speed intervals considered where I1 (0–1 cm/s), I2 (1–5 cm/s), I3 (5–10 cm/s), I4 (10–15 cm/s), I5 (15–20 cm/s), I6 (20–25 cm/s), I7 (25–30 cm/s), I8 (30–35 cm/s), I9 (35–40 cm/s), and I10 (40–90 cm/s). We recorded the highest speed interval that the mouse ran for at least 3 s (Vmax3s) and assigned as value of the test the lower speed of the interval (e.g., 10 for I4 and 35 cm/s for I9).
The animal procedures followed the 2010/63/EU directive on the protection of animals used for scientific purposes and were approved by the Ethic Committee on Animal Use of INRCA.
Statistical analysis
Differential patterns of survival due to the treatment were estimated by Kaplan-Meier and Cox-regression taking also into account sex. Differences in physical performances (strength score, track length, activity and Vmax3s) and anthropometric measurements (weight, surface temperature) were investigated by generalized linear mixed models (SPSS 25.0). Linear mixed models were used to take into account the longitudinal design of the study in mice and to manage missing values arising by increasing death events with age [26]. The identifier of each mouse, experimental group, sex, and time (months) as well as the interaction between experimental group and age were included in the models. A model using age as continuous variable was used to identify significant changes among experimental groups, while another model using age as categorical variable was used for graphical representations and to investigate pairwise comparisons (sequential Bonferroni) at different time periods. The final linear models were developed after testing various repeated covariance types and variable distributions and choosing the combination that minimize the Akaike’s Information Criteria. The Satterthwaite approximation and robust estimator were used to take into account unbalanced data and violation of the assumptions.
Results
Commercial C60-OO has unpredictable concentration and activity
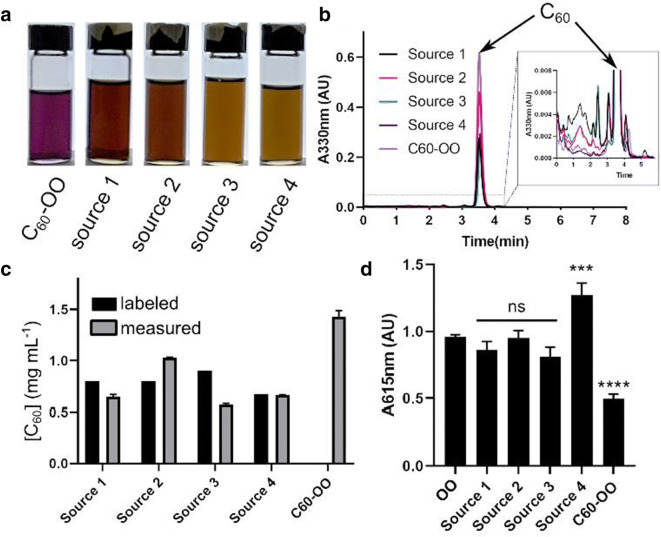
We first obtained samples C60-OO from 4 popular online sources (labeled Source 1–4) and formulated pristine C60-OO in-house. C60-OO should be a transparent deep purple (f. (f.1a).1a). However, there was marked discrepancy in the visual appearance of the commercial formulations, appearing dark brown and almost black (Fig. (Fig.1a).1a). We performed HPLC on the samples and found a notable increase in impurity peaks in the commercial samples (Fig. (Fig.1b,1b, inset). Again, with HPLC, we measured the concentration of C60 in each sample and compared it to the claimed concentration of the label. We again found remarkable variability in C60 concentration and in deviation from the stated concentration. The highest concentration found was in the freshly made in-house Pristine C60-OO at 1.4 mg mL-1, with the lowest being Source 3, at 0.57 mg mL−1 (Fig. (Fig.1c).1c). When we compared these concentrations with the claimed concentrations on the labels, we found deviations ranging from negligible for Source 4, to > 38.5% for Source 2, with all except Source 4 deviating by > 18%.
Basic characterization of C60-OO preparations. a Photographs of commercial C60-OO preparations as received from the manufacturer in comparison to pristine C60-OO. b Representative HPLC chromatograms of extracted C60 from C60-OO preparations. Inset is zoomed to aid in visualization of impurity peaks. Note the decreased peak height of the main C60 peak and increased non-C60 impurity peaks seen in the commercially sourced C60-OO. c Measured C60 concentrations in C60-OO samples relative to their label claims. In-house C60-OO is shown without a labeled concentration. Data are mean ± SEM. d ROS protection activity of C60-OO preparations as measured by 4-NBP assay. Only in-house pristine C60-OO exhibits protection. Data are mean ± SEM. Data were analyzed with one-way ANOVA with Bonferroni post-hoc correction. ***p < 0.001, ****p < 0.0001, ns = not significant
Because the presumed mechanistic basis for the biological activity of C60-OO is protecting biomolecules from ROS damage, we tested the C60-OO formulations for their ability to protect olive oil from forced peroxidation/epoxidation in vitro [27, 28]. Briefly, C60-OO formulations were diluted in identical olive oil to the same concentration and subjected to forced oxidation with H2O2/acetic acid/sulfuric acid, washed with water to remove the H2O2/acetic acid/sulfuric acid, and then assayed for reactive alkylating compounds with 4-(4-nitrobenzyl)pyridine (4-NBP) (Fig. (Fig.1d).1d). As expected, pristine C60-OO was able to significantly reduce the 4-NBP signal, indicating that it significantly protected the OO from oxidative damage. However, the commercial formulations showed less activity, with no commercial source causing significantly lower signal relative to the OO control. Unexpectedly, Source 4 showed more signal than olive oil only control, indicating not just a lack of protection, but the presence of additional reactive compounds.
Light-exposed C60-OO is toxic in mice
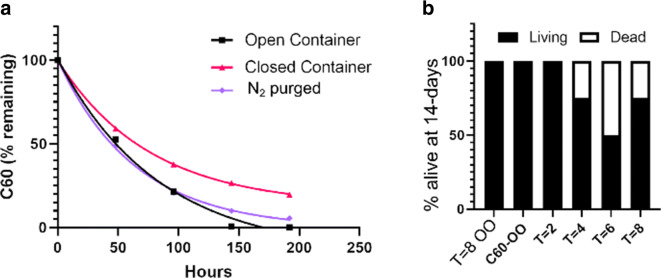
While several studies have established the lack of acute toxicity of C60 when freshly prepared or handled in laboratory environments, the toxicity of C60-OO when handled long-term under conditions of chronic typical use is much less well established. To simulate how C60-OO might reasonably be stored and handled in the hands of the end-consumer in an accelerated fashion, we continuously stirred pristine C60-OO either open to the air, sealed with an air atmosphere, or sealed with a nitrogen purged atmosphere in transparent glass vials, and exposed the samples to a fluorescent lamp that approximates sunlight (7 × 104 lux, 380–700 nm). Unmodified olive oil was used as a negative control. Every 48 h, we took a sample and measured the C60 concentration by HPLC. Regardless of the storage condition, the samples degraded at approximately the same rate with t1/2 of 2 days, indicating that C60-OO will degrade over time if exposed to light (Fig. (Fig.2a).2a). From this experiment, we saved aliquots taken at t = 0–8 days from the open air vial, and administered a single dose of 4 mg kg−1 (or the equivalent volume of oil based on original sample concentration of 1.4 mg mL−1) to 20-week-old C57BL6/J mice via IP injection, and monitored for 14 days. A significant effect was seen on the interaction of weight and time with the experimental group which received C60-OO which had been exposed to illumination for 8 days (p < 0.05) by a repeated measured mixed effect model. No other group demonstrated significant weight loss compared to the control group, which received olive oil which had received 8 days of illuminated prior to injection. (Supplementary Figure 2, panel A). As expected, neither olive oil alone nor pristine C60-OO at 0 and 2 days showed toxicity. However, at 4, 6, and 8 days, 25%, 50%, and 25% of the mice died, respectively, indicating a light-dependent cause of mortality (Fig. (Fig.2b).2b). Upon necropsy, mice dosed with pristine C60-OO exhibited no observable abnormal phenotypes (t = 0). However, most mice dosed with irradiated C60-OO displayed obvious pathology with increasing severity with increasing irradiation (Supplementary Table 1). Mice given an IP injection of irradiated olive oil alone showed a 25% prevalence of irritated small intestines, enlarged pancreas, and abdominal masses. Mice that were dosed with irradiated C60-OO were found to have numerous adhesions in the intestinal cavity, dilated small intestines, enlarged livers and spleens, and extensive fibrin formation, further substantiating a light-dependent toxicity. Together, these results indicate that the C60 in C60-OO degrades when exposed to light, and that toxic species can develop that can cause morbidity and mortality in mice.
Light-induced toxic byproduct formation of C60-OO. a Degradation of pristine C60-OO by light under the indicated atmosphere calculated by AUC of the C60 peak on HPLC. Data were fit with single-exponential decays. t1/2 were as follows: b Mice remaining alive at the end of the 14-day observation period with samples taken from the open-container sample shown in a at the given time interval shown in days. T = 8 OO indicates OO only exposed to light for 8 days. n = 4 per group
IP injections of pristine C60-OO does not extend lifespan in CB6F1 aged mice
In order to test whether pristine C60-OO can increase lifespan, we randomized aged female CB6F1 mice (25–27.3 months, 26 months on average. n = 11 per group) into C60-OO test or OO control groups. CB6F1 mice were selected because of their increased genetic diversity relative to the C57BL/6 J mice. We hypothesized that IP administration might display an increased benefit compared to oral administration as the penetration of C60 in the tissues could be enhanced by this route of administration. Hence, C60-OO mice were treated with 1.7 mg kg−1 C60 by IP injection using insulin syringes daily for the first week of the study, then weekly for seven weeks, then fortnightly for twenty more weeks, for a total of 25 injections (dosing schedule mimicking the oral administration described previously [14] and with volume-matched OO alone), and survival was monitored (Fig. (Fig.3).3). There were no significant differences in weight loss over the course of the study (Supplementary Figure 2, panel B). The curves did not resolve, with median survival of 145 weeks for the olive oil only control group, and 144 weeks for the C60-OO group (p = 0.87, log-rank test). Even when only the initial dosing period is considered (through week 28; 925 days), there fails to be any statistical difference between the data sets. Thus, we do not find a significant effect of IP injections of C60-OO on lifespan in our aged CB6F1 mouse population.
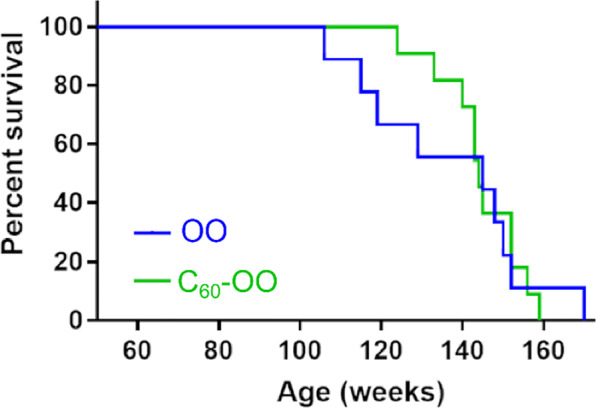
Lifespan study with IP injections of C60 in aged mice CB6F1 mice. There was no significant difference in mice treated with C60-OO vs OO alone. Data show survival curves estimated by Kaplan-Meier of female CB6F1 mice randomly distributed into two groups of 11 mice and starting the treatment at 100–109 weeks of age
Replication of lifespan study combined with health span assessment in C57BL/6 J mice
We also replicated the lifespan study in C57BL/6 J mice with oral administration. In order to minimize the potential toxicity of the treatment, in this replication study, we used EVOO (extra virgin olive oil). EVOO is a source of phenolic compounds with antioxidant activity that may help to prevent degradation of C60-OO. EVOO was selected as a carrier because the cold-press extraction method used in EVOO production generates fewer oxidation products than heat-extracted OO under normal storage conditions [29, 30]. We exactly replicated the dosing and the administration strategy proposed in the original study of C60 in rats using separate randomized groups of adult and old C57BL/6 J mice in C60-EVOO or EVOO alone groups [14]. The adult mice groups supplemented with C60-EVOO or EVOO are named C60-EVOO_Y or EVOO_Y, whereas old mice supplemented with C60-EVOO or EVOO are named C60-EVOO_O or EVOO_O. The respective numbers of mice and the design of the study is reported in Supplementary table 2. Survival, as well as health span data, was compared with those of untreated control mice starting from the age of 21 months.
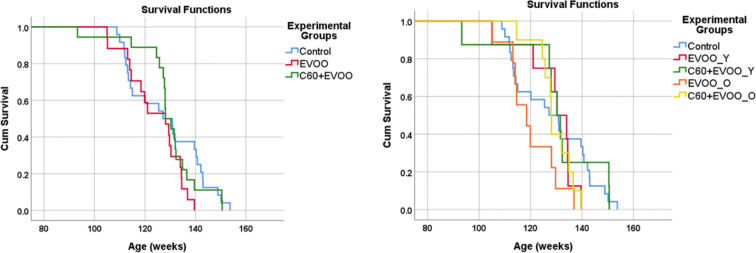
Kaplan-Meir analysis failed to detect significant differences (Log-Rank: p = 0.202; Breslow: p = 0.461; Tarone-Ware: p = 0.360) between C60-EVOO and EVOO groups versus controls, and the same result was obtained (Log-Rank: p = 0.132; Breslow: p = 0.320; Tarone-Ware: p = 0.246) considering all five experimental groups (EVOO_Y, EVOO_O, C60-EVOO_Y, C60-EVOO_O, and controls). Survival curves obtained from Kaplan-Meier analysis are displayed in Fig. Fig.4.4. The median lifespan in weeks (95% CI) estimated by Kaplan-Meier were as follows: Controls = 127 (114–140); C60-EVOO_Y = 130 (125–135); EVOO_Y = 130 (123–136); C60-EVOO_O = 128 (127–128); EVOO_O = 118 (107–129). We additionally did not observe differences in survival adjusting the Kaplan-Meier for sex (Supplementary Figure 1) or by cox-regression (Wald = 3.13 and p = 0.209 for Experimental Groups).
Replication of lifespan study with oral supplementation of C60 in C57BL/6 J. Left panel: survival curves estimated by Kaplan-Meier in all C57BL/6 J mice treated with C60-EVOO or EVOO versus untreated controls. Right panel: survival curves of the same mice subdivided in those starting the treatment with C60-EVOO or EVOO at the age of 9 months (C60 + EVOO_Y and EVOO_Y, respectively) and those starting the treatment at the age of 23 months (C60 + EVOO_O and EVOO_Y, respectively) versus untreated mice (Control). No significant differences in survival were detected among the experimental groups and between females and male mice. Analysis of survival curves starts at 21 months of age for all experimental groups. Respective numbers of mice at start are as follows: Controls n = 24 (12 F, 12 M); EVOO_Y n = 8 (4 F, 4 M); C60-EVOO_Y n = 8 (3 F, 5 M); EVOO_O n = 9 (5F, 4 M); C60-EVOO_O n = 10 (5F, 5 M)
Health span assessment was investigated by linear mixed models including data of mice from the 21st to the 30th month of life (see Supplementary Table 2 for the respective sample size). The analysis performed using age as categorical or continuous variable provided evidence of negligible beneficial effects of the treatments. The mean estimates obtained from the analysis using age as categorical variable are shown in Fig. Fig.5,5, while results obtained using age as continuous variable are reported in Supplementary tables 3–8).
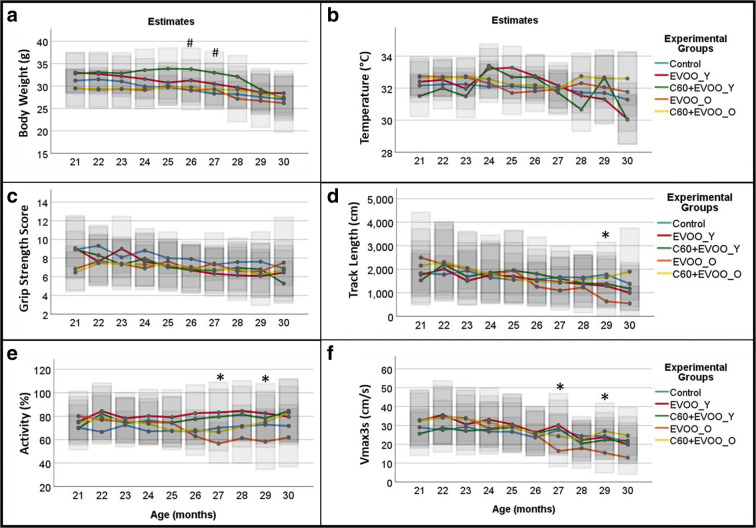
Health span assessment in C57BL/6 J. Graphical presentation of age-related changes of body weight (panel a), surface temperature (panel b), grip strength score (panel c) as well as of three parameters obtained from a 5-min locomotor activity test [track length (panel d), activity (panel e) and max speed kept for 3 s (Vmax3s, panel f)] in C57BL/6 J mice. C60 + EVOO_Y and EVOO_Y represent the groups of mice starting the treatment with C60-EVOO or EVOO, respectively, at the age of 9 months. C60 + EVOO_O and EVOO_O represent the groups of mice starting the treatment with C60-EVOO or EVOO, respectively, at the age of 23 months. The graphs are plotted using the mean estimates obtained by linear mixed models using age (months) as categorical (ordinal) variable, sex (categorical), experimental group (categorical) and the interaction between age and experimental groups. Transparent grey intervals overlaid with the means represents 95% confidence interval. Pairwise comparisons (sequential Bonferroni) are shown only for those experimental groups displaying significant changes from controls also in linear mixed model using age as continuous variables. # p < 005 for C60 + EVOO_Y compared to controls. * p < 0.05 for EVOO_O compared to controls. Comparison of health span data starts at 21 months of age. Respective numbers of mice at 21 months are as follows: Controls n = 24 (12 F, 12 M); EVOO_Y n = 8 (4 F, 4 M); C60-EVOO_Y n = 8 (3 F, 5 M); EVOO_O n = 9 (5F, 4 M); C60-EVOO_O n = 10 (5F, 5 M). Since the number of mice decreases with aging due to age-related mortality, a detailed description of the sample size is reported in Supplementary table 2
The C60-EVOO_Y group displayed higher weight compared to controls for a limited period of time (Fig. (Fig.5,5, panel a). The analysis performed including age as continuous variable (Supplementary table 3) confirmed this data (p = 0.046) albeit at a marginal significance. Moreover, the interaction between weight and age for C60-EVOO_Y group was not significantly different from controls, suggesting a similar pattern during age. We also did not observe a significant difference between the EVOO_O, EVOO_Y, and the C60-EVOO_Y groups compared to controls (Supplementary table 3). Considering the whole population, males were heavier than females (Coeff. 0.164, p < 0.001, supplementary table 3) and the weight decreased with age (Coeff. − 0.015, p < 0.001, supplementary table 3) as expected in old mice.
Surface temperature of the mice displayed a high variability, likely related to circadian cycle and differences in room temperature, and we did not detect any significant difference among treated groups and controls using age as categorical (Fig. (Fig.1,1, panel b) or continuous variable (Supplementary table 4). Considering the whole population, male surface temperature was less than females (Coeff. − 0.032, p < 0.001, supplementary table 3) and the temperature slightly decreased with age (Coeff. − 0.002, p < 0.039, supplementary table 3) confirming previous observations of body core temperature in different strain of mice [31, 32].
A general decline with age was also observed in the grip strength score (Supplementary table 5). We also detected a marginal significant difference for the EVOO_O group (p = 0.046 compared to controls), which was not confirmed including age as categorical variable in the model (Fig. (Fig.5,5, panel c).
Regarding the data obtained from locomotor activity, such as Track length, Activity, and Vmax3s, we detected only significant changes in the EVOO_O group compared to controls (Fig. (Fig.5,5, panels d–f, and supplementary tables 6–8). Interestingly, for all these assessments, the EVOO displayed a faster decline compared to the controls, as suggested by the negative coefficient of the interaction term (Supplementary table 6–8) and by the significant pairwise comparisons detected above 26 months of age (Fig. (Fig.5,5, panels d–f). None of the locomotor activity variables displayed sex differences, while only Vmax3s displayed a marked decline with age between 21 and 30 months (Coeff. − 0.030, p < 0.018, supplementary table 3).
Discussion
The lack of acute toxicity of C60 is supported by multiple pre-clinical studies and its efficacy in life extension lifespan has both mechanistic rationale [33] and evidence in at least one animal model [14]. We conducted a lifespan study with IP injections in aged female CB6F1 mice and found no difference in lifespan between C60-OO and OO control. We also replicated the study in adult and old (females and males) C57BL/6 J mice by oral supplementation with C60-EVOO or EVOO without observing differences in lifespan compared to control. Our results ran counter to previously reported data seen in rats, indicating that the observed life extension effect is not present in mice when treatment begin later in life. Supporting health span measurements (obtained by a battery of physical performance tests and anthropometric data) also did not indicate any effects of C60-OO and EVOO. While the age and sex differences in weight and temperature are in line with those expected during aging mice [31], the difference in weight detected in the C60-EVOO_Y group compared to controls can explained through factors unrelated to the effect of treatment. This difference can likely be attributed to baseline differences that have been amplified as a result of reduced sample size with advancing age, alongside an increase in weight variability due to the presence of ascites with cancer origin causing increased weight gain in a subset of the population. This is supported by the fact that we failed to identify significant differences in the pattern between weight and age compared to controls or between similar sample sets.
Conversely, the lower performances displayed by the EVOO_O group in the locomotor activity tests (track length, activity and Vmax3s) suggest the presence of a slightly faster functional decline of this group compared to controls. This is partly confirmed by the lowest median survival of this group (albeit not significant compared to controls). From these data, it might be possible to argue that the sensitivity of some health span measurements revealed a faster functional decline induced by EVOO supplementation in old age. However, significant differences in locomotor activity data versus controls were observed only at sporadic months, when the sample size of this group was drastically reduced. In contrast with the claimed health benefit of EVOO and previously reported lifespan studies performed in nematodes, we failed to detect any significant benefit of EVOO on the lifespan of laboratory mice [34, 35]. However, it should be considered that the standard diets of laboratory mice are already optimized and enriched with essential nutrients and antioxidants and that we supplemented the mice with EVOO for a very limited period.
As a result of the reported lifespan effects on mammals, C60-OO has become available from several unregulated online sources and is currently consumed by people hoping to reap the alleged lifespan benefit. However, the assumption of safety and efficacy is predicated on the assumptions that the products being provided to consumers are consistently and verifiably as high quality as those produced in laboratory environments and that the increase of lifespan previously observed in rats is valid in other species independently by the age. Our analysis of C60 produced by online vendors found marked variation between vendors in visual appearance, concentration, and biochemical activity. We further confirm a lack of acute toxicity in C60-OO in mice at 4 mL kg−1, but that substantial degradation of C60-OO occurs with light, and that this degradation results in profoundly toxic species capable of causing death in mice in days to weeks after a single dose. Multiple samples C60-OO were procured from online vendors, and each supplied a product which was visibly discolored when compared to a freshly produced sample of C60-OO. Most of the samples deviate from the labelled concentration of C60, contain numerous additional components by HPLC, and show limited evidence of antioxidant activity.
Our toxicity study is limited in that our tests used a rapid degradation model using high doses of C60-OO. However, the light level the samples were exposed to are well within the realm of typical experience (direct normal luminescence from the sun ~ 1 × 105 lux vs experimental luminescence of 0.7 × 105 lux ) and the endpoint that we used—death in less than 2 weeks—is also an extreme form of toxicity. We effectively demonstrate that photodegradation of C60-OO generates toxic species, with some treatment groups experiencing as high as a 50% death rate and plainly evident pathological abnormalities in the 14-day toxicity study that are not present in pristine C60-OO, in which we observed no deaths or pathological findings in the 14-day toxicity study and nearly identical lifespans to control mice in a lifespan study. We do not know the identity of these toxic species or what levels are required to cause harm, particularly with chronic consumption. However, the observed toxicity raises concern for long-term health effects because it is often thought of as a supplement that should be taken consumed regularly and will likely be stored for long periods in household settings. These observations, alongside the lack of effectiveness in our lifespan studies, indicate that C60-OO preparations are of little or negligible benefit to preventing age related mortality under the best of conditions and may generate toxic photodegradation products under household storage conditions.
In our lifespan studies, we used different routes of administration. IP injections of C60 were tested in aged CB6F1 female mice and oral administration was tested in adult and old C57BL/6 J mice. Since olive oil and many other oils may form lipid granulomas when administered by IP [36], it may be hypothesized that the lack of efficacy observed in the lifespan study on CB6F1 mice may be affected by this phenomenon. However, the mean lifespan of treated and untreated mice were comparable to lifespans reported by others for CB6F1 mice (126–143 weeks) [37], and even with the oral supplementation, we have not been able to demonstrate a clear effect on lifespan. In support of the idea that lipid granulomas were not a significant cause of the acute lethality of these injections, previous works have demonstrated that injections of similar volumes of olive oil had relatively little ill effect [38, 39]. Since the publication of the effects of C60 on the lifespan of rats [14], no other survival study has been published with C60; thus, our study is the first to address this topic in mice. As C60 is poorly soluble in water, many authors have given preference to its modified derivatives. Polyhydroxylated fullerene slowed down aging of the nematode Caenorhabditis elegans [40], while carboxyfullerene significantly extended lifespan (albeit at much lower extent than the increase shown in rats with C60) and improved cognition in mice [41]. However, the biological properties of modified fullerenes can be very different form C60. For example, carboxyfullerenes and other derivatives may act as superoxide dismutase mimetics [41], a property that cannot be attributed to C60. Hence, the observed lifespan benefits of fullerene derivatives cannot be extended to C60. While we used the same weight-adjusted dosing regimen of the original study in rats [14], this was not meant as a repeat of those experiments, but rather a test if the life-extension effect is generalizable to aging mice. Our results indicate that it is not. It has to be underlined that our sample size was based on the large difference in survival previously observed in rats and it is not enough to provide evidence for a slightly effect on survival. It is also important to highlight that the original study in rats was performed in males while our study with oral supplementation was performed in a combined group of males and females. We have not addressed in details sex differences because of the low power to make those comparisons but we have performed a stratified analysis (supplementary figure 1) that confirms any lack of efficacy. Hence, the profound life extension observed in rats seen by others [14] does not seem to be recapitulated in our rodent model, and calls into question the generalizability of the biological benefit of C60-OO in aging.
Conclusions
C60 is a unique chemical entity with many potential applications in chemistry and biology. A prior observation that consuming C60-OO drastically enhances lifespan in rats [14] and an apparent lack of acute toxicity of C60 has led to excitement in some communities regarding the potential for those results to translate across species. Our results call into question the generalizability of the efficacy data across different species and age ranges. Even with C60-EVOO maintained under laboratory conditions, no significant effect on mean lifespan or functional performance in C57BL/6 J mice was seen compared to control groups. As well, the putative therapeutic benefit of C60-OO is further undercut by the apparent propensity for the material to degrade via photo-oxidation during storage. Samples of C60-OO exposed to direct light progressively lost C60 as monitored by HPLC, and thereafter demonstrated toxic effect when injected into a small cohort of mice. Toxicity of C60 products should be more extensively assessed if the material is to be considered for downstream therapeutic purposes.
Acknowledgments
The authors would like to thank SUNY ESF for support in the use of their facilities and Onondaga Community College (OCC) for the lending of equipment. This work was funded under a sponsored research agreement from BioSenex, Ltd., by Ricerca Corrente funding from Italian Ministry of Health to IRCCS INRCA, as well as through generous grants from Longecity.org and the Methuselah Foundation.
Compliance with ethical standards
The animal procedures followed the 2010/63/EU directive on the protection of animals used for scientific purposes and were approved by the Ethic Committee on Animal Use of INRCA.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kelsey J. Moody and Marco Malavolta shared senior authorship.