- Journal List
- Elsevier Sponsored Documents
- PMC6130051
Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism
Clea Bárcena
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
2Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
Pedro M. Quirós
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
2Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
Sylvère Durand
3Cell Biology and Metabolomics Platforms, Gustave Roussy Cancer Campus, Villejuif, France
4Equipe 11 labellisée par la Ligue contre le Cancer, Centre de Recherche des Cordeliers, Paris, France
5INSERM, U1138, Paris, France
6Université Paris Descartes, Sorbonne Paris Cité, Paris, France
Pablo Mayoral
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
Francisco Rodríguez
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
Xurde M. Caravia
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
Guillermo Mariño
7Grupo Autofagia y Metabolismo, Instituto de Investigación Sanitaria del Principado de Asturias (IISPA), Oviedo, Spain
8Departamento de Biología Funcional, Facultad de Medicina, Universidad de Oviedo, 33006 Oviedo, Spain
Cecilia Garabaya
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
María Teresa Fernández-García
9Unidad de Histopatología Molecular, IUOPA, Universidad de Oviedo, 33006 Oviedo, Spain
Guido Kroemer
3Cell Biology and Metabolomics Platforms, Gustave Roussy Cancer Campus, Villejuif, France
4Equipe 11 labellisée par la Ligue contre le Cancer, Centre de Recherche des Cordeliers, Paris, France
5INSERM, U1138, Paris, France
6Université Paris Descartes, Sorbonne Paris Cité, Paris, France
10Université Pierre et Marie Curie, Paris, France
11Pôle de Biologie, Hôpital Européen Georges Pompidou, AP-HP, Paris, France
12Karolinska Institute, Department of Women’s and Children’s Health, Karolinska University Hospital, Stockholm, Sweden
José M.P. Freije
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
2Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
Carlos López-Otín
1Departamento de Bioquímica y Biología Molecular, Facultad de Medicina, Instituto Universitario de Oncología (IUOPA), Universidad de Oviedo, 33006 Oviedo, Spain
2Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain
Associated Data
- Supplementary Materials
- Video S1. Improved Health Span in Zmpste24−/− Mice by Cholic Acid Treatment, Related to Figure 6 Zmpste24−/− control mice (left) show an aggravated loss of hair, hind atrophy and loss of movement than Zmpste24−/− mice supplemented with cholic acid (CA) (right).mmc4.mp4 (30M)GUID: 9AD0C356-54FC-48DC-AB06-B83D10132B57Document S1. Supplemental Experimental Procedures, Figures S1–S6, and Table S3mmc1.pdf (984K)GUID: B2D875A1-8F74-40F1-9295-014DD29D6D85Table S1. Untargeted Metabolomics of Liver from WT and LmnaG609G/G609G Mice under a Control and a Methionine Restriction Diet, Related to Figures 4 and 5mmc2.xlsx (44K)GUID: D8903D0E-554B-4E9E-A042-B641E42BFA4BTable S2. Targeted Metabolomics of Bile Acids of Liver and Ileum from WT and LmnaG609G/G609G Mice under a Control and a Methionine Restriction Diet and from Zmpste24−/− Mice under a Control Diet, Related to Figures 5 and 6mmc3.xlsx (20K)GUID: 5C693697-5352-4A01-9A07-42DCF0E729E3Document S2. Article plus Supplemental Informationmmc5.pdf (4.0M)GUID: EC04B033-5908-469D-807F-43CECDDFD733
Summary
Dietary intervention constitutes a feasible approach for modulating metabolism and improving the health span and lifespan. Methionine restriction (MR) delays the appearance of age-related diseases and increases longevity in normal mice. However, the effect of MR on premature aging remains to be elucidated. Here, we describe that MR extends lifespan in two different mouse models of Hutchinson-Gilford progeria syndrome (HGPS) by reversing the transcriptome alterations in inflammation and DNA-damage response genes present in this condition. Further, MR improves the lipid profile and changes bile acid levels and conjugation, both in wild-type and in progeroid mice. Notably, treatment with cholic acid improves the health span and lifespan in vivo. These results suggest the existence of a metabolic pathway involved in the longevity extension achieved by MR and support the possibility of dietary interventions for treating progeria.
Introduction
Over the past years, multiple studies have confirmed the idea that caloric restriction (CR), i.e., a reduction of caloric intake without malnutrition, can delay aging in numerous model organisms (Fontana and Partridge, 2015, Madeo et al., 2015). Thus, CR has been proven to act efficiently on several hallmarks of aging, enhancing metabolic flexibility, stem cell function, and DNA repair and reducing inflammation and age-related diseases (Finkel, 2015, López-Otín et al., 2013, López-Otín et al., 2016, Mitchell et al., 2016). Activation of AMPK, SIRT1, and the autophagy response and suppression of the growth hormone (GH)/IGF1 and mTOR signaling pathways have been implicated in the extension of the health span observed under CR (Breese et al., 1991, Burkewitz et al., 2014, Dunn et al., 1997, Fontana et al., 2016, Galluzzi et al., 2014, Johnson et al., 2013, Mercken et al., 2014, Stenesen et al., 2013). Recently, it has been described that CR extends the lifespan in a DNA-repair-deficient mouse model by reducing genomic stress (Vermeij et al., 2016). However, the underlying molecular pathways at the core of CR interventions remain incompletely defined. In this regard, the study of macronutrient restriction accomplished by the reduction of specific constituents of the diet instead of a simple and non-specific decrease in calorie intake has gained attention in recent years. Thus, restriction of proteins or certain amino acids in the diet can reduce the incidence of age-associated diseases and simultaneously increase lifespan (Mirzaei et al., 2014, Nakagawa et al., 2012, Solon-Biet et al., 2015). Among these, methionine restriction (MR) is supposed to exert its benefits, at least in part, through the suppression of the GH/IGF1 somatotrophic axis (Miller et al., 2005). MR also modulates mitochondrial activity, which, in turn, leads to an increase in both respiration rate (an increment of VO2 and VCO2) and energy expenditure (Orgeron et al., 2014) and increases the levels of hydrogen sulfide (H2S), a molecule that is indispensable for lifespan extension under CR (Hine et al., 2015).
The effects of amino acid restriction have been assessed in normal aging yet remain unexplored in genetic models of accelerated aging. Hutchinson-Gilford progeria syndrome (HGPS) is a rare premature aging condition in which a point mutation in the LMNA gene causes the accumulation at the nuclear envelope of an aberrant precursor of lamin A, named progerin (Eriksson et al., 2003, De Sandre-Giovannoli et al., 2003). Children with HGPS manifest growth impairment, lipodystrophy, and dermal and bone abnormalities, as well as cardiovascular alterations that lead to an average life expectancy of 13 years (Gordon et al., 2014). Of note, during normal human aging, ever-increasing amounts of progerin are produced as a result of LMNA aberrant alternative splicing, suggesting that progerin not only participates in the pathogenesis of HGPS but may also contribute to normal aging (Burtner and Kennedy, 2010, Scaffidi and Misteli, 2006). We have generated a mouse model carrying the equivalent of the most common human HGPS-associated mutation, p.Gly608Gly (p.Gly609Gly in mice). This model phenocopies most alterations observed in children with HGPS (Osorio et al., 2011). So far, the main manipulations that have led to an improvement of fitness and extension of lifespan in the p.Gly609Gly mouse model of HGPS have been a morpholino-based therapy that reduces progerin accumulation (Osorio et al., 2011), genetic or pharmacological attenuation of inflammation (Osorio et al., 2012), interruption of lamin A-progerin binding (Lee et al., 2016), inhibition of the acetyltransferase NAT10 (Balmus et al., 2018), and in vivo activation of reprogramming (Ocampo et al., 2016).
Here, we report that a low-methionine diet can extend health span and lifespan in the HGPS mouse model by ameliorating the alterations in signaling pathways,such as inflammation or DNA damage. Moreover, we describe that MR improves metabolic homeostasis and restores lipid and bile acid (BA) levels, and that treatment with cholic acid improves health span and lifespan in a mouse model of progeria. Together, our results suggest that the use of dietary interventions can effectively influence the metabolic deregulation of patients affected by accelerated aging syndromes, providing beneficial effects that could improve their quality of life and extend their longevity.
Results
Methionine-Restricted Diet Extends Lifespan in a Mouse Model of HGPS
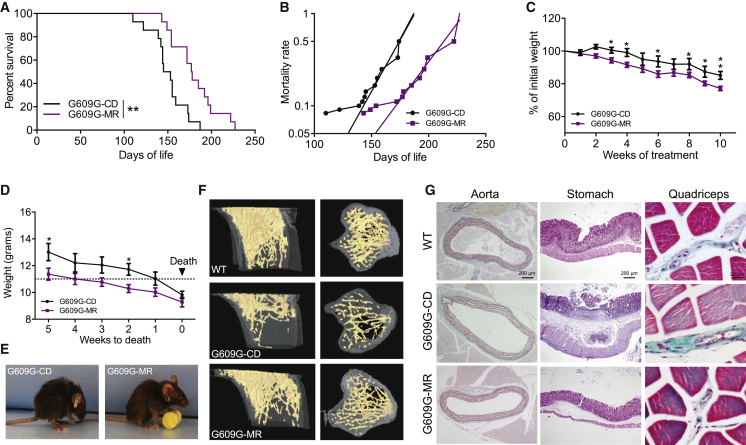
Mice homozygous for the HGPS mutation (LmnaG609G/G609G in a C57BL/6N background) were fed a diet with a low concentration (0.12%) of methionine. This dietary intervention reduced mortality rate and extended the median lifespan in both male and female LmnaG609G/G609G progeroid mice by 20% (Figures 1A, 1B, S1A, and S1B). Also, LmnaG609G/G609G mice fed an MR diet showed a tendency toward an increased maximum survival by approximately 20% (Figures 1A and S1C). As compared to wild-type (WT) controls, LmnaG609G/G609G mice on a control diet (CD) (LmnaG609G/G609G-CD mice) are characterized by a relatively small size and low body weight (Osorio et al., 2011). Administration of the MR diet to LmnaG609G/G609G mice further exacerbated these characteristics (Figure 1C). Although LmnaG609G/G609G-CD mice usually died by the time they reached a weight below 11 g, mice on the MR diet managed to survive with a lower weight for several weeks (Figure 1D), suggesting that small body size is not an obligatory predictor of health span in progeria. Indeed, MR retarded the appearance of lordokyphosis and loss of grooming in LmnaG609G/G609G mice (Figure 1E). LmnaG609G/G609G mice showed a basal loss of bone tissue due to inflammation-associated osteolysis, which is reversed under pharmacological and genetic inhibition of inflammation (Osorio et al., 2012). As expected, LmnaG609G/G609G mice with a CD showed a clear loss of bone tissue (Figures 1F and S1D). However, with an MR diet, we found an extensive amelioration of the phenotype with an almost complete recovery of bone structure. MR in LmnaG609G/G609G mice enhanced bone volume, number and connectivity of trabeculae, and bone mineral density in tibia, reaching values close to the normal ones found in WT animals (Figures 1F and S1D). Histological analysis showed that LmnaG609G/G609G-CD mice had aortic alterations such as focal fibrosis with mild chronic infiltrate and an increment of matrix between elastic fibers. These alterations were not present in LmnaG609G/G609G-MR mice (Figure 1G). Also, although both LmnaG609G/G609G mice on a CD and those on an MR diet had atrophy of the gastric mucosa, LmnaG609G/G609G-CD mice presented glandular dilatations and ulcerations (Figure 1G). At the skeletal muscle, quadriceps, LmnaG609G/G609G-CD mice had focal and perivascular fibrosis. Again, these alterations were absent in mice that were fed an MR diet (Figure 1G).
MR Enhances Lifespan and Health Span in LmnaG609G/G609G Mice
(A) Survival plot of LmnaG609G/G609G mice fed an MR diet (purple) or control diet (black) (n = 14 per group). Survival curves were analyzed with the log-rank (Mantel-Cox) test (p = 0.0023) and Gehan-Breslow-Wilcoxon test (p = 0.0044).
(B) Mice fed an MR diet show a lower mortality rate. Slope of G609G-CD is 0.022, and slope of G609G-MR is 0.016. Curve comparison: p < 0.001.
(C) MR diet in LmnaG609G/G609G mice induces a smaller weight. ∗p < 0.05 (CD, n = 11; MR, n = 12).
(D) Weight loss during the 5 weeks before death. LmnaG609G/G609G-CD mice die by the time they reach a weight lower than 11 g, while LmnaG609G/G609G-MR mice survive a lower weight. ∗p < 0.05 (n = 6 per group).
(E) LmnaG609G/G609G-MR mice present a deceleration of the aged phenotype, appreciated by a retardation of lordokyphosis and a longer maintenance of grooming.
(F) Three-dimensional longitudinal and transversal images of tibias from WT-CD, LmnaG609G/G609G-CD, and LmnaG609G/G609G-MR littermate mice, generated with μCT analysis (n = 3 per group).
(G) Histological analysis of the aorta stained with orcein technique, gastric mucosa stained with H&E, and skeletal muscle (quadriceps) stained with H&E and Gomori trichrome.
Error bars indicate SEM. Scale bars are indicated in each figure. See also Figure S1.
Analysis of Classical CR-Modulated Longevity Pathways in MR Progeria Mice
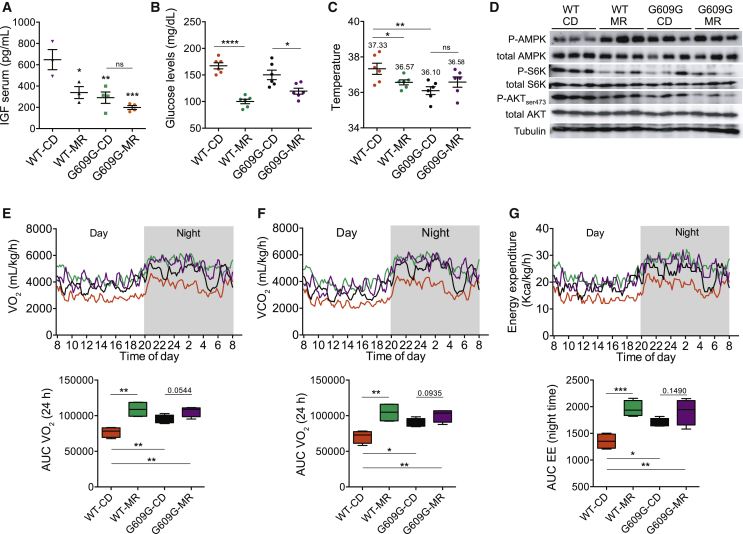
Similar to other mouse models of premature aging (Mariño et al., 2010, Niedernhofer et al., 2006, van der Pluijm et al., 2007), LmnaG609G/G609G mice display constitutive inhibition of the GH/IGF1 (insulin growth factor 1) pathway (Figure 2A), although an MR diet decreased glycemia under fasting conditions in both genotypes (Figure 2B). Reminiscent of published results on CR (Duffy et al., 1990, Heilbronn et al., 2006), MR caused a reduction in body temperature in WT mice. When compared to WT controls, however, LmnaG609G/G609G mice that were kept on a CD exhibited reduced body temperature (Figure 2C). Hence, a general reduction of metabolic turnover cannot explain the beneficial effect of MR on the progeroid phenotype. Also, although an MR diet enhanced AMPK and reduced mTORC1 activity in liver from WT mice (Figures 2D and S2), similarly to another model of HGPS (Mariño et al., 2008), LmnaG609G/G609G-CD mice exhibited basal AMPK activation—demonstrated by its hyperphosphorylation—and an inhibition of mTORC1 in liver compared to WT mice, indicated by the reduced phosphorylation of its targets P70-S6K and AKTSer473 (Figures 2D and S2). Of note, an MR diet induced a further decrease of AKTSer473 phosphorylation in LmnaG609G/G609G mice, without changes in P70-S6K (Figures 2D and S2).
MR Fails to Induce the Classical Survival Response in LmnaG609G/G609G Mice
(A) Reduced basal levels of IGF1 in sera from LmnaG609G/G609G mice (WT, n = 3 per group; G609G = 4 per group).
(B) Glucose levels after a 5-hr fasting in the week 6 of treatment (n = 6 per group).
(C) Rectal temperature in week 6 of treatment (n = 6 per group).
For (A)–(C), lines in dot plots indicate mean ± SEM.
(D) Western blot analysis of phosphorylated (P)-AMPK, total AMPK, P-P70S6K, total P70S6K, P-AKT (ser473), and total AKT in liver protein extracts. The Western blots shown were carried out with the same samples run in parallel in three blots: one for P- and total (T)-AMPK, one for P- and T-P70S6K, and a third blot for P- and T-AKT and α-tubulin. All membranes were stained with Ponceau to confirm equal protein loading and homogeneous transfer.
(E–G). Indirect calorimetry. (E) VO2 consumption (milliliters per kilogram per hour); (F) VCO2 production (milliliters per kilogram per hour); and (G) energy expenditure (kilocalories per kilogram per hour) during 24 hr (n = 4 per group). Quantification of the area under the curve (AUC) of each parameter is provided below in boxplots (n = 4). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
See also Figure S2.
MR promotes an increase in respiration rates (VO2 and VCO2) and energy expenditure, features that were observed in WT mice fed an MR diet (Figures 2E–2G). On a CD, LmnaG609G/G609G mice exhibited a significant increment in respiration rates and energy expenditure when compared to WT animals (Figures 2E–2G), which could be explained as a possible strategy to increase thermogenesis. MR only slightly increased these values in LmnaG609G/G609G mice (Figures 2E–2G). On the basis of these results, we conclude that LmnaG609G/G609G mice have a basal activation of the pro-survival mechanisms of MR previously described, involving the GH/IGF1 axis, the AMPK/mTORC1 energy sensors, thermoregulation, or general effects on energy expenditure. Therefore, the positive effects of MR on these progeroid mice cannot be explained exclusively by the classical CR pathways and should involve additional mechanisms.
MR Attenuates the Transcriptome Alterations of Progeroid Mice
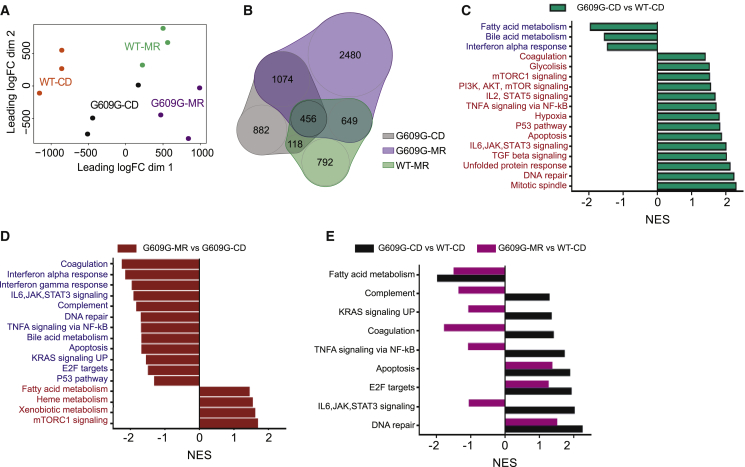
To gain insight into the molecular mechanisms by which an MR diet extends lifespan in progeroid mice, we analyzed the transcriptome of liver tissues from fasted LmnaG609G/G609G and WT mice kept on a CD or MR diet. We selected this tissue because of its central metabolic function. Principal-component analysis indicated clear distinctions between the two genotypes and the two diets (Figure 3A). When compared to WT-CD, most transcripts altered in WT-MR mice were similarly changed in LmnaG609G/G609G-MR mice. By contrast, there were more than 900 genes whose expression was altered in LmnaG609G/G609G-CD but not in LmnaG609G/G609G-MR mice, as compared to that from WT-CD mice (Figure 3B). Additionally, around 2,500 transcripts were changed in LmnaG609G/G609G-MR mice and not in LmnaG609G/G609G-CD when compared to WT-CD (Figure 3B), confirming a shift of the transcriptome landscape due to the dietary modification. Gene set enrichment analysis (GSEA) confirmed previously reported differences in transcript levels between LmnaG609G/G609G and WT mice, including those associated with the hyperactivation of inflammation and DNA-damage response pathways (Osorio et al., 2011, Osorio et al., 2012), together with some undescribed downregulated pathways such as fatty acid metabolism and BA metabolism (Figure 3C). By comparing the transcript levels of LmnaG609G/G609G-MR and LmnaG609G/G609G-CD, we found that some of the pathways that were upregulated in progeroid models were repressed by MR in LmnaG609G/G609G mice. This applies to several groups of genes related to inflammation, such as the interferon alpha and gamma response, tumor necrosis factor alpha (TNFA) signaling via nuclear factor κB (NF-κB), and interleukin-6 (IL-6), JAK, and STAT3 signaling. Also, DNA repair, BA metabolism, apoptosis, and p53 pathways were reduced by MR in LmnaG609G/G609G mice (Figure 3D).
MR Restores the Transcriptome of LmnaG609G/G609G Mice
(A) Multidimensional scaling showed that the individual cases of our dataset were grouped according to the corresponding genotype and diet.
(B) Proportional Venn diagram illustrating that most of the transcripts modified by the MR diet were shared between LmnaG609G/G609G and WT mice.
(C and D) GSEA NES analysis of (C) LmnaG609G/G609G -CD versus WT-CD and (D) LmnaG609G/G609G -CD versus LmnaG609G/G609G-MR mice. Red indicates upregulated pathways, and blue indicates downregulated pathways.
(E) GSEA NES comparison of LmnaG609G/G609G-CD mice versus WT-CD mice and LmnaG609G/G609G-MR mice versus WT-CD mice.
See also Figure S3.
Next, we compared the transcriptome profiles of LmnaG609G/G609G mice on either a CD or an MR diet with those of WT mice on a CD. Several pathways that are altered in the LmnaG609G/G609G-CD mice with respect to WT controls were completely or partially rescued by MR (Figure 3E), as indicated by the fact that the GSEA-normalized enrichment score (NES) for LmnaG609G/G609G-MR mice was opposite to the NES for LmnaG609G/G609G-CD mice. This applies for IL-6, JAK, and STAT3 signaling, TNFA signaling via NF-κB, coagulation, complement, and KRAS signaling, indicating that MR was able to counteract progeria-associated changes in these pathways. For other pathways, such as fatty acid metabolism, apoptosis, E2F targets, and DNA repair, the NES values showed a partial improvement of the progeroid transcriptome upon MR (Figure 3E).
To identify possible genotype-specific responses, we performed GSEA of transcript levels from WT-MR versus WT-CD mice (Figure S3A). Most pathways were altered in the same direction as in LmnaG609G/G609G-MR mice. Thus, mTORC1 signaling and xenobiotic, fatty acid and heme metabolism pathways were upregulated, whereas coagulation, complement, and interferon alpha and gamma response signaling were downregulated by MR. However, several other pathways were affected by MR in WT and LmnaG609G/G609G mice in opposite directions. This applied to pathways related to DNA damage (p53 pathway, DNA repair, and apoptosis) and inflammation (TNFA signaling via NF-kB and IL-6, JAK, STAT3 signaling), which were increased by MR in WT mice yet reduced in LmnaG609G/G609G mice. This difference might be related to the basal hyperactivation of these pathways in LmnaG609G/G609G mice on a CD (Figure 3C) (Osorio et al., 2011, Osorio et al., 2012). Collectively, the aforementioned results indicate that MR attenuates or reverts several of the transcriptome alterations that accompany the phenotypic changes resulting from the LmnaG609G/G609G mutation, especially those accounting for inflammation and DNA-damage signaling.
MR Normalizes the Metabolome and Improves the Lipid Profile in the HGPS Mouse Model
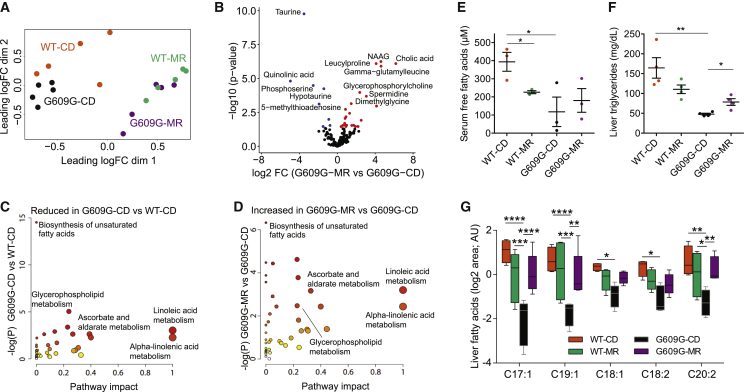
To deepen the study of the changes induced by MR in both WT and progeroid mice, we performed mass spectrometric metabolome profiling in livers from fasted WT and LmnaG609G/G609G mice kept on a CD or an MR diet. This revealed clear genotype and diet effects, as determined by multidimensional scaling (Figure 4A) and unsupervised hierarchical clustering and heatmap analysis (Figure S4A). These results closely resemble those derived from the analysis of transcript levels, suggesting that the dietary intervention has more impact on metabolism than the Lmna mutation.
MR Restores Metabolome and Lipid Levels in LmnaG609G/G609G Mice
(A) Multidimensional scaling of the samples from the metabolomics analysis grouped according to the corresponding genotype and diet.
(B) Volcano plot showing the most differentially changed metabolites in LmnaG609G/G609-MR compared to LmnaG609G/G609G-CD mice. Upregulated metabolites are indicated in red, and downregulated metabolites are indicated in blue.
(C and D) Metabolic enrichment analysis showing (C) pathways downregulated in LmnaG609G/G609G-CD compared to WT-CD mice and (D) pathways upregulated in LmnaG609G/G609G-MR compared to LmnaG609G/G609G-CD mice.
(E) Serum free fatty acid levels (in micromolar) (n = 3 per group).
(F) Liver triglyceride levels (n = 5 per group).
For (E) and (F), lines in dot plots indicate mean ± SEM.
(G) Relative liver levels of MUFAs (heptadecenoic (C17:1), nonadecenoic (C19:1) and oleic acid (C18:1)) and PUFAs (linoleic (C18:2) and eicosadienoic acid (C20:2)) in WT-CD, WT-MR, LmnaG609G/G609G-CD, and LmnaG609G/G609G-MR mice (WT, n = 4 per group; G609G, n = 5 per group). Levels represent the log2 of the normalized area in a.u. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Examination of individual metabolites showed that MR depleted the methionine derivatives taurine, hypotaurine, and 5-methylthioadenosine. Thus, MR caused a 20-fold downregulation of taurine in WT and an 11-fold downregulation in LmnaG609G/G609G livers, together with an increment in glycine (Figures 4B, S4B, and S4C). MR also reduced quinolinic acid and phosphoserine (Figures 4B and S4B). Conversely, MR caused an increase in the polyamine spermidine by 10-fold in LmnaG609G/G609G livers (Figure 4B). Along with the increment in spermidine, MR caused an increase in some modified amino acids such as leucylproline and γ-glutamylleucine, intermediates of protein catabolism, as well as in dimethylglycine, which participates in choline and methionine metabolism (Figures 4B and S4B). MR also increased glycerophosphorylcholine, a choline derivative that plays a major role in osmotic balance (Figure 4B). In summary, an assessment of individual metabolites suggests that MR attenuates the difference between the metabolic landscapes from WT and LmnaG609G/G609G mice, inducing similar metabolic changes in both phenotypes.
Enrichment analyses using MetaboAnalyst led to the identification of pathways that were downregulated in LmnaG609G/G609G samples as compared to WT controls and that were reinstated by MR in the progeroid mice: biosynthesis of unsaturated fatty acids, glycerophospholipid metabolism, linoleic and alpha-linolenic acid metabolism, and ascorbate and aldarate metabolism (Figures 4C and 4D). Remarkably, in the aforementioned microarray analysis, we found that the expression of genes involved in fatty acid metabolism was reduced in LmnaG609G/G609G-CD mice when compared to WT-CD mice and was partially recovered in LmnaG609G/G609G-MR mice (Figures 3C and 3E). Among the molecules that were most reduced in LmnaG609G/G609G-CD mice compared to WT-CD mice, we detected several fatty acids, such as palmitoleic, linoleic, oleic, and heptadecenoic acids (Figure S4D). Accordingly, we found that global serum free fatty acids and liver triglycerides were reduced in LmnaG609G/G609G-CD mice and partly recovered on an MR diet (Figures 4E and 4F). MR also caused the recovery of several polyunsaturated (PUFAs) and monounsaturated fatty acids (MUFAs) in LmnaG609G/G609G mice, keeping their levels similar to those in WT-MR mice (Figure 4G). Altogether, we conclude that MR modifies the lipid profile in LmnaG609G/G609G mice and restores lipid metabolic pathways that were repressed in progeroid mice.
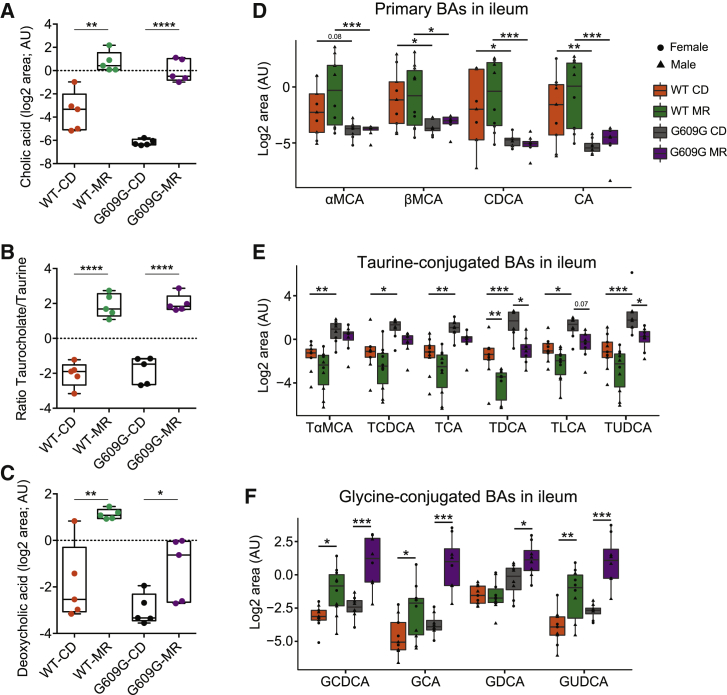
MR in Progeroid Mice Increases BA Levels in Liver under Fasting Conditions and Reverts the High Levels of Taurine Conjugation
In the microarray analysis described earlier, we also noted differences in BA metabolism at the transcriptional level in LmnaG609G/G609G mice under a CD when compared to both WT-CD and LmnaG609G/G609G-MR mice (Figures 3C and 3D). The metabolomic profiling further allowed us to explore this alteration, which revealed that cholic acid was downregulated (11 times) in LmnaG609G/G609G-CD mice versus WT-CD mice (Figure S4D) yet upregulated by 7 and 12 times in LmnaG609G/G609G-MR and WT-MR mice, respectively (Figure 5A). Notably, cholic acid was upregulated 85-fold in LmnaG609G/G609G-MR mice versus LmnaG609G/G609G-CD mice, being the metabolite most induced by MR (Figures 4B and S4B). As shown earlier, taurine was severely downregulated in progeroid mice under MR (Figures 4B and S4C); however, BAs conjugated with taurine, such as taurocholic or taurodeoxycholic acid, were not changed upon MR in liver (Figures S5A and S5B). This implied that the ratio of taurocholic acid to taurine was remarkably upregulated in both LmnaG609G/G609G and WT mice maintained on MR (Figure 5B). The secondary BA deoxycholic acid was also decreased in LmnaG609G/G609G-CD mice versus WT-CD mice and increased upon MR in both LmnaG609G/G609G and WT mice (Figure 5C), thus exhibiting a pattern of modulation similar to that of cholic acid.
Profiling BA Levels in Fasting and Re-feeding Conditions Shows a Loss of Primary BAs in LmnaG609G/G609G Mice and an Abnormal Increment in Taurine Conjugation
(A–C) Boxplots showing the (A) levels of cholic acid, (B) taurocholate/taurine ratio, and (C) levels of deoxycholic acid under fasting conditions in liver samples from WT-CD, WT-MR, LmnaG609G/G609G-CD and LmnaG609G/G609G-MR (n = 5 per group). Levels of cholic and deoxycholic acid are presented as the log2 of the normalized area in a.u. (AU).
(D–F) Boxplots showing the levels of (D) primary BAs, (E) taurine-conjugated BAs, and (F) glycine-conjugated BAs in ileum samples from WT-CD, WT-MR, LmnaG609G/G609G-CD and LmnaG609G/G609G-MR mice under refeeding conditions (n = 8 per group). Levels represent the log2 of the normalized area in a.u. Sex is indicated with different symbols. αMCA, α-muricholic acid; βMCA, β-muricholic acid; CDCA, chenodeoxycholic acid; CA, cholic acid; TαMCA, tauro-α-muricholic acid; TCDCA, taurochenodeoxycholic acid; TCA, taurocholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; GDCA, glycodeoxycholic acid; GUDCA, glycoursodeoxycholic acid.
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. See also Figure S5 and Table S2.
To understand the alteration of BAs in LmnaG609G/G609G mice, we examined the mRNA expression levels of enzymes involved in their generation, including those encoding components of the cytochrome P450 complex (Lorbek et al., 2012). mRNA expression analysis of Cyp7a1, Cyp7b1, Cyp8b1, Cyp3a11, and Cyp39a1 in liver showed that the only enzyme consistently activated in both WT and progeroid mice fed an MR diet was Cyp39a1 (Figure S5C). Of note, Cyp39a1 is involved in alternative pathways of BA synthesis (Lorbek et al., 2012); in contrast, Cyp7b1 and Cyp8b1, key enzymes in the classical and alternative synthesis pathway of BAs, were repressed under an MR diet (Figure S5C). These results suggest that the increment in BAs observed under an MR diet does not depend on an increment of the synthesis pathways described so far.
To deepen the origin of the changes observed in BAs in WT and progeroid mice on a CD and an MR diet, we profiled BA levels in liver and ileum through targeted metabolomics under refeeding conditions, when BA synthesis is induced. We observed that, under this nutritional condition, levels of primary BAs in ileum were reduced in progeroid mice on both a CD and an MR diet (Figure 5D); however, this trend was not observed in liver (Figure S5D). These results suggest that the increment in primary BAs observed in liver in MR-fed mice under fasting conditions was not dependent on their synthesis. Indeed, no differences were observed in the transcript levels of key enzymes involved in the generation of BAs upon refeeding conditions (Figure S5E). Remarkably, and similar to fasting conditions, Cyp39a1 was the only enzyme induced by an MR diet in both genotypes (Figure S5E). LmnaG609G/G609G-CD mice had abnormally high levels of BAs conjugated with taurine in ileum of both sexes (Figure 5E). On an MR diet, the levels of taurine-conjugated BAs were lower (Figure 5E), probably due to the taurine deficiency associated with the modified diet (Figure S4C). Conversely, both WT and LmnaG609G/G609G mice fed the MR diet showed an increment in glycine-conjugated BAs, such as glycochenodeoxycholic acid (GCDCA), glycocholic acid (GCA), glycodeoxycholic acid (GDCA), and glycoursodeoxycholic acid (GUDCA) (Figure 5F). Glycine-conjugated BAs, which are usually found in much lower levels than taurine-conjugated BAs, are not synthetized in mouse liver but generated by the gut microbiome in the ileum. Collectively, these results prove an alteration in the conjugation of BAs, with an excess of taurine conjugation in LmnaG609G/G609G-CD mice that is reversed in an MR diet, favoring this diet as an increment in glycine-conjugated BAs.
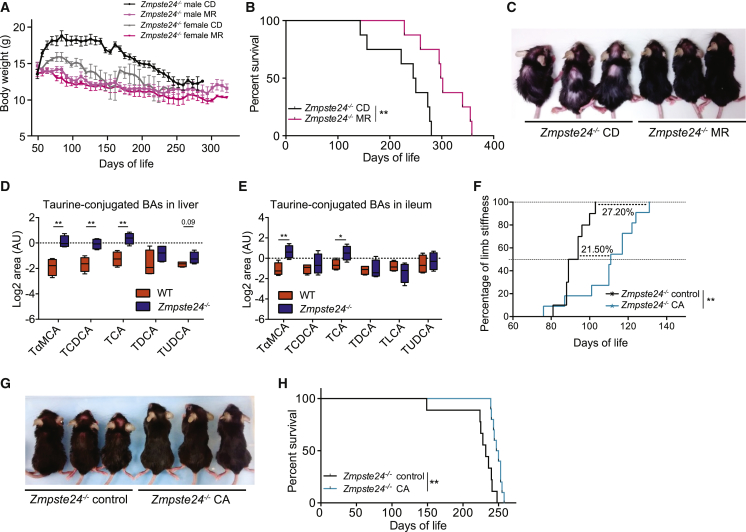
MR Diet and In Vivo BA Supplementation Extends Health Span and Lifespan in Zmpste24 Null Progeroid Mice
We explored whether the effects of MR observed in our LmnaG609G/G609G progeroid model could be reproduced in a different mouse model of premature aging and whether the alterations in BAs were also shared by different progeroid mice. Thus, we first evaluated the effect of MR in the Zmpste24−/− mouse model, deficient in the protease ZMPSTE24, which processes prelamin A into mature lamin A (Navarro et al., 2005, Pendás et al., 2002). As observed in LmnaG609G/G609G mice, Zmpste24−/− under MR (Zmpste24−/− MR) also showed improvements in health and survival. Despite their reduced size (Figure 6A), Zmpste24−/− MR mice had a 21% increase in median survival and an almost 28% increase in maximal survival (Figures 6B and S6A) and showed a healthier aspect, mostly apparent by a reduced loss of hair and improved atrophy of hindlimbs (Figures 6C and S6B).
Both MR and In Vivo Modulation of BA Pool Exerts Beneficial Effects in Zmpste24−/− Mouse Model of Progeria
(A) Zmpste24−/− mice fed an MR diet (light and dark pink) show a lower weight than Zmpste24−/− mice under a CD (black and gray) (n = 4 per group).
(B) Survival plot of Zmpste24−/− mice fed an MR diet (pink) or CD (black) (n = 8 per group). Survival curves were analyzed with the log-rank (Mantel-Cox) test (p = 0.0029) and Gehan-Breslow-Wilcoxon test (p = 0.008).
(C) Zmpste24−/− MR mice show a healthier aspect, manifested by a reduced loss of hair and improved cervicothoracic lordokyphosis, than Zmpste24−/− CD mice.
(D and E) Taurine-conjugated BAs in (D) liver and (E) ileum samples from WT and Zmpste24−/− female mice (n = 4 per group). Levels represent the log2 of the normalized area in a.u. TαMCA, tauro-α-muricholic acid; TCDCA, taurochenodeoxycholic acid; TCA, taurocholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid.
(F) Zmpste24−/− mice fed a diet enriched in cholic acid (Zmpste24−/− CA) show a retarded manifestation of the phenotype-associated hindlimb stiffness compared to Zmpste24−/− mice fed the CD (Zmpste24−/− control). Appearance of limb stiffness was analyzed with the log-rank (Mantel-Cox) test (p = 0.001) and Gehan-Breslow-Wilcoxon test (p = 0.0089) (n = 10 per group).
(G) Zmpste24−/− CA mice (right) show a healthier aspect, manifested by a bigger size, reduced loss of hair, and improved cervicothoracic lordokyphosis, compared to Zmpste24−/− control mice (left).
(H) Survival plot of Zmpste24−/− CA mice (n = 10) and Zmpste24−/− control mice (n = 10). Survival curves were analyzed with the log-rank (Mantel-Cox) test (p = 0.0011) and Gehan-Breslow-Wilcoxon test (p = 0.0012).
Targeted metabolomics showed no differences in the levels of primary BAs in liver and ileum of Zmpste24−/− mice under re-feeding conditions in a CD (Figures S6C and S6D); however, and similarly to LmnaG609G/G609G mice, these progeroid mice showed a significant increment in taurine conjugation (Figures 6D and 6E). These results indicate that progeroid mice have a common increment of taurine-conjugated BAs. MR shifted BA conjugation from an increase in taurine- to glycine-conjugated BAs (Figures 5E and 5F), which is independent of changes in primary BA synthesis after refeeding, opposite to the increase in primary BAs observed in fasting conditions. Based on these results, we hypothesize that BA supplementation could normalize BA levels and improve their health span. Therefore, we treated our Zmpste24−/− progeroid model, where health span is easier to assess than in LmnaG609G/G609G due to their longer survival, with the main primary BA, cholic acid. Zmpste24−/− mice kept on a diet enriched with 0.1% cholic acid (Zmpste24−/− CA) had a delayed appearance of the phenotype-associated hindlimb stiffness and a consequent improvement in their daily movement when compared to Zmpste24−/− mice fed a normal diet (Zmpste24−/− control) (Figures 6F and S6E; Video S1). Also, Zmpste24−/− CA had a milder loss of weight during the progression of the phenotype (Figure S6F). The healthier status of CA-treated progeroid mice was also assessed by their improved cervicothoracic lordokyphosis, reduced loss of hair, and bigger size (Figure 6G), as well as by their enhanced median survival (7%; Figure 6H) and tendency to an enhanced maximal survival (Figure S6G).
Zmpste24−/− control mice (left) show an aggravated loss of hair, hind atrophy and loss of movement than Zmpste24−/− mice supplemented with cholic acid (CA) (right).
Collectively, these results indicate that MR promotes a healthier phenotype and extends survival in two different mouse models of progeria. Additionally, we have proved that in vivo supplementation of cholic acid in the diet exerts beneficial effects in progeria, suggesting that modulation of BA metabolism could have a key role regulating longevity in mice.
Discussion
CR is the most effective intervention to prolong lifespan in diverse organisms so far; however, its application in humans presents important barriers, as food deprivation is something that not many people would accept to practice. This has led to the development of CR mimetics (Madeo et al., 2014, Mercken et al., 2012), although their use has limitations owing to the diverse effects of CR throughout different tissues (Igarashi and Guarente, 2016). In contrast, MR appears to be a simpler way of effectively acting on metabolism; more importantly, it offers a translational strategy that is easier to apply to humans (Mann et al., 1999, McCarty et al., 2009, Schmidt et al., 2016). The simplicity of a modified diet and the lack of negative secondary effects make this type of intervention a promising tool for future treatments of human diseases.
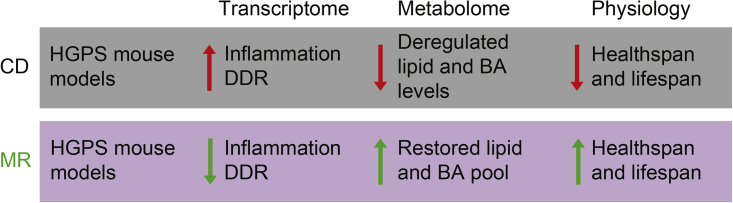
In the present work, we demonstrate that an MR diet can substantially rescue the pathologic phenotype observed in both the LmnaG609G/G609G and the Zmpste24−/− HGPS mouse models. With an MR diet, we found an amelioration of the most severely modified pathways, such as DNA repair and inflammation, and the normalization of certain metabolic alterations (Figure 7). Among the last, we detected a recovery in metabolic pathways related to lipid metabolism, supporting the role of lipid homeostasis in longevity (Schroeder and Brunet, 2015). Also, we observed that LmnaG609G/G609G mice have reduced levels of primary and secondary BAs in liver and that MR induces an increment of them in both WT and progeroid mice. Additionally, we found that both LmnaG609G/G609G and Zmpste24−/− progeroid mice have abnormally high levels of taurine-conjugated BAs. Probably due to the deficiency in taurine under MR (Elshorbagy et al., 2010), mice fed with this diet have reduced taurine conjugation with increased glycine conjugation. As a result, the excess of taurine-conjugated BAs observed in progeroid mice is reverted. We also showed that the addition of 0.1% cholic acid in the diet has a beneficial outcome in both the health span and lifespan of an HGPS mouse model. Previous studies have suggested a role of BAs in the promotion of health and longevity in different organisms, from lower eukaryotes to mammals. BAs—and, in particular, lithocholic acid—extend longevity in yeast in an mTOR-independent manner (Arlia-Ciommo et al., 2014, Goldberg et al., 2010, Leonov et al., 2017). Also, loss of the bile-acid-like steroids called dafachronic acids reduces fitness and lifespan in C. elegans (Magner et al., 2013). Conversely, in the long-lived Ghrhr−/− mice, there is an increase in BA levels in bile, serum, and liver (Amador-Noguez et al., 2007). In our study, we observed that MR can substantially rescue the phenotype of the HGPS mouse, and we suggest that this occurs, at least in part, by modulating the BA profile. However, the exact mechanism by which MR affects BAs levels and improves health span and lifespan remains to be elucidated.
Effect of MR in Progeroid Mice
Mouse models of HGPS, such as LmnaG609G/G609G and Zmpste24−/−, show increased systemic inflammation and hyperactivation of the DNA damage response (DDR) pathways when mice are maintained on a CD. The LmnaG609G/G609G mouse model also shows a deregulation of both lipid and BA levels. MR decreases the transcriptional activation of those stress pathways, restores lipid levels, and changes BA levels and conjugation. The attenuation of both transcriptional and metabolic alterations by MR increases health span and lifespan in both progeroid mouse models.
Overall, we provide evidence that diet modulation can effectively increase health and the lifespan, not only in a normal physiological situation but also in two mouse models of accelerated aging, hence opening the possibility for implementing diet-based strategies for the treatment of these diseases.
Experimental Procedures
Additional details and resources used in this work can be found in the Supplemental Experimental Procedures.
Mouse Models
LmnaG609G/G609G and Zmpste24−/− mice in a C57BL/6N background were generated by crossing heterozygous mice and genotyped in our laboratory as previously described (Osorio et al., 2011, Varela et al., 2005). Mice were bred in a specific pathogen-free (SPF) area, and the experiments were carried out in a conventional area with exclusion barriers. Mice were caged separately by sex and genotype and were checked daily for water and food supplies, as well as for good physical condition. Experiments with modified diets were initiated at 7 weeks of age. For the microarray analysis and untargeted metabolomics under fasting conditions, 6 males per group were sacrificed after 6 hr of fasting at 110 days of life and used to obtain serum and tissue samples. For the BA profiling under refeeding conditions, mice were fasted overnight and then allowed to feed ad libitum for 4 hr before being sacrificed. All animal experiments were approved by the Committee for Animal Experimentation of the Universidad de Oviedo and were performed in accordance with its guidelines, making every effort to minimize the suffering of the mice.
Diet Treatment
Rodent diets used in this study were acquired from Research Diets. The control diet (A11051302) contains a 0.86% of methionine, whereas the methionine-restricted diet (A11051301) contains 0.12% methionine. Cystine was not added to the diets during their elaboration. Methionine-restricted and control diets were provided that combined solid and crushed pellets in all experimental groups to facilitate the feeding of the mutant mice. A diet enriched in cholic acid was prepared with a dried ground global diet (Envigo #2014S); it was mixed with filtered water (0.8 mL/g) containing, in suspension, cholic acid (Sigma, #C1129) to obtain a final concentration of 0.1% in the resulting diet. The mixture was kneaded to form pellets that were allowed to dry overnight in a hood, resulting in small pellets of soft consistency.
Histological Analysis
Tissues were fixed in 4% paraformaldehyde in PBS and stored in 70% ethanol. Fixed tissues were embedded in paraffin by standard procedures. Blocks were sectioned (5 μm) and stained with H&E (stomach), orcein (aorta), or H&E with Gomori trichrome (muscle).
Analysis of Bone Structure
All tibia samples were scanned by high-resolution micro-computed tomography (SkyScan 1174, SkyScan, Kontich, Belgium). The parameters were measured according to the American Society for Bone and Mineral Research (ASBMR) histomorphometry nomenclature (Parfitt et al., 1987).
Metabolic and Movement Measurements
Metabolic parameters such as VO2, VCO2 and energy expenditure, as well as daily movement, were obtained using the CLAMS Comprehensive Lab Animal Monitoring System (Oxymax CLAMS, Columbus Instruments) and analyzed following the manufacturer’s instructions. Mice were monitored for 48 hr, and the first 24 hr were discarded in the analysis, as this was considered an acclimation period.
Western Blot Analysis
Tissues were collected and immediately frozen in dry ice. Samples were processed for western blotting using standard methods. Antibodies against phospho-P70S6K (Thr421/Ser424) (#9204), total P70S6K (#2708), phospho-AKT (Ser473) (#9271), total AKT (#9272), phospho-AMPKα (#2531), and total AMPKα (#2532) were obtained from Cell Signaling Technology, and α-Tubulin (T6074) was obtained from Sigma.
IGF1 Analysis, Serum Fatty Acids, and Liver Triglycerides
IGF1 measurements were performed in serum with EDTA using an IGF1 ELISA kit obtained from R&D Systems (MG100). Free fatty acids in serum were assayed using the luminometric Free Fatty Acid Assay Kit from Abnova (KA1667). Triglycerides in liver were assayed using the EnzyChrom Triglyceride Assay Kit from BioAssay Systems (ETGA-200).
Microarray Profiling
Microarray profiling was performed as previously described (Osorio et al., 2011), using a GeneChip Mouse Gene 1.0 ST Array (Affymetrix). Raw data were processed with the RMAExpress program (http://RMAExpress.bmbolstad.com), using default settings. Differentially expressed genes in each condition were identified using the R/Bioconductor package limma (Ritchie et al., 2015). A proportional Venn diagram was generated using nVenn (http://degradome.uniovi.es/cgi-bin/nVenn/nvenn.cgi) (Pérez-Silva et al., 2018). GSEA v2.2.0 and MSigDB release 5.1 (http://software.broadinstitute.org/gsea/index.jsp) were used for pathway enrichment analysis. Weighted enrichment scores were calculated with gene expression lists ranked by signal-to-noise ratio (maximum gene set size: 500; minimum gene set size: 20; number of permutations: 1,000; gene set database: Hallmark; false discovery rate [FDR] ⩽ 0.25; and p ⩽ 0.01). Plots representing GSEA NESs were generated using GraphPad Prism 6.0.
Untargeted and Targeted Metabolomics Analysis
Metabolites were extracted from 30 mg of each tissue using a mix of cold methanol/water/chloroform (9:1:1). Samples were homogenized, and phase separation was performed by centrifugation. For untargeted metabolomics, supernatants were collected and evaporated, and dried extracts were solubilized in methanol. Two aliquots were produced for gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) analysis. For targeted metabolomics, collected supernatants were evaporated, dried, and solubilized with MilliQ water. One aliquot was transferred to LC vials and injected into LC-MS. Targeted metabolomics were performed using ultra-high-pressure liquid chromatography coupled to mass spectrometry (UHPLC-MS). Analytical methods and data processing were performed as previously described (Enot et al., 2015). Results were represented as the normalized area of the MS picks in log2 scale using a.u. Normalization was performed by correcting the area of the MS picks across the batches using the quality control (QC) pooled samples (regularly injected during the analysis) and by centering their values around the mean of the QC areas. Differentially expressed metabolites were identified using the R/Bioconductor package limma (Ritchie et al., 2015). Metabolic pathway analysis was performed with MetaboAnalyst 3.0 (http://www.metaboanalyst.ca/) (Xia et al., 2015), using the hypergeometric test for representation analysis, and relative-betweenness centrality was selected for pathway topology analysis. For targeted metabolomics, statistical differences in BAs were calculated using a one-way ANOVA with Tukey’s multiple comparison post hoc test, including sex as cofactor.
Statistical Analysis
Unless otherwise specified, all experimental data are reported as mean ± SEM, except for the boxplots that were generated using Tukey’s method. Statistical differences were calculated using two-tailed Student’s t test for pairwise comparisons between two groups and one-way ANOVA with Tukey’s correction for multiple comparisons for more than two groups. Survival analysis was performed by using the Kaplan-Meier method, and statistical differences were analyzed with the log-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. Power analysis of survival was 80%. Mortality rate was calculated using the Gompertz model. Statistical differences between mortality curves was calculated using the extra-sum-of-squares F test. Maximal survival was analyzed using one-tailed Fisher’s exact test at the 80th percentile (Wang et al., 2004). All statistical tests, data analysis, and plots were generated using R and RStudio (R Core Team, Vienna, Austria, https://www.r-project.org; RStudio Team, Boston, MA, USA, https://www.rstudio.com) and GraphPad Prism 6.0. Plots and figures were modified using Adobe Illustrator CC.
Acknowledgments
We thank Drs. V. Quesada, A. Fueyo, A.R. Folgueras, G. Velasco, M. Mittelbrunn, and J. M. Fraile for helpful comments and advice; and R. Feijoo, A. Moyano, and S. Alvarez-Miranda for excellent technical assistance. We also thank M.S. Pitiot, V. García de la Fuente, M.C. Muñiz, V. Loredo, and E. Pascual for histological and micro-computed tomography (μCT) analysis. We also acknowledge the generous support by J.I. Cabrera. The Instituto Universitario de Oncología is supported by the Fundación Bancaria Caja de Ahorros de Asturias. C.L.-O. is supported by grants from the European Union (ERC-2016-ADG, DeAge); Ministerio de Economía y Competitividad (MINECO/FEDER: SAF2014-52413-R and SAF2017-87655-R); Instituto de Salud Carlos III (RTICC); Progeria Research Foundation (PRF2016-66); and EDP Foundation. J.M.P.F. is supported by the Ministerio de Economía y Competitividad (MINECO/FEDER: SAF2015-64157-R) and Gobierno del Principado de Asturias. G.M. is funded by the Ramón y Cajal Program (RYC-2013-12751) and supported by the Ministerio de Economía y Competitividad (MINECO/FEDER: BFU2015-68539) and BBVA Foundation (BBM_BIO_3105). G.K. is supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR) – Projets Blancs; ANR under the frame of E-Rare-2; the ERA-Net for Research on Rare Diseases; Association pour la Recherche sur le Cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Institut Universitaire de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LeDucq Foundation; the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Author Contributions
C.B., P.M.Q., C.L.-O., and J.M.P.F. conceived and designed experiments. C.B., P.M.Q., P.M., F.R., X.M.C., G.M., and C.G. performed experiments and analyzed data. S.D. and G.K. performed metabolomics. M.T.F.-G. performed histopathological analysis. C.B., C.L.-O., and J.M.P.F. wrote the manuscript, and P.M.Q. and G.K. contributed to the editing and proofreading.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, three tables, and one video and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.07.089.
Data and Software Availability
The accession number for the microarray data and raw files reported in this paper is GEO: {"type":"entrez-geo","attrs":{"text":"GSE117188","term_id":"117188"}}GSE117188.