Highlights
- •An IHF extends lifespan in male rats and flies
- •The IHF decreases the profiles of FFAs in serum and multiple tissues in rats and flies
- •The IHF downregulates anabolism of FFAs and upregulates catabolism of FFAs
- •Decreased FFAs upregulate PPRC1, mediating the effect of IHF on lifespan via PPARG
Summary
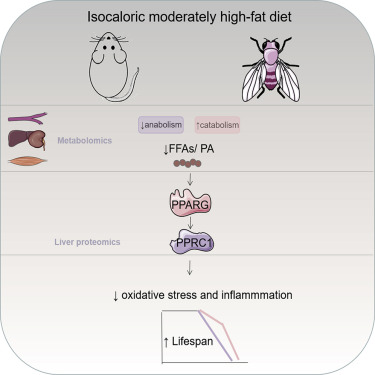
Graphical abstract
Keywords
Introduction
- Dehghan M.
- Mente A.
- Zhang X.
- Swaminathan S.
- Li W.
- Mohan V.
- Iqbal R.
- Kumar R.
- Wentzel-Viljoen E.
- Rosengren A.
- et al.
Results
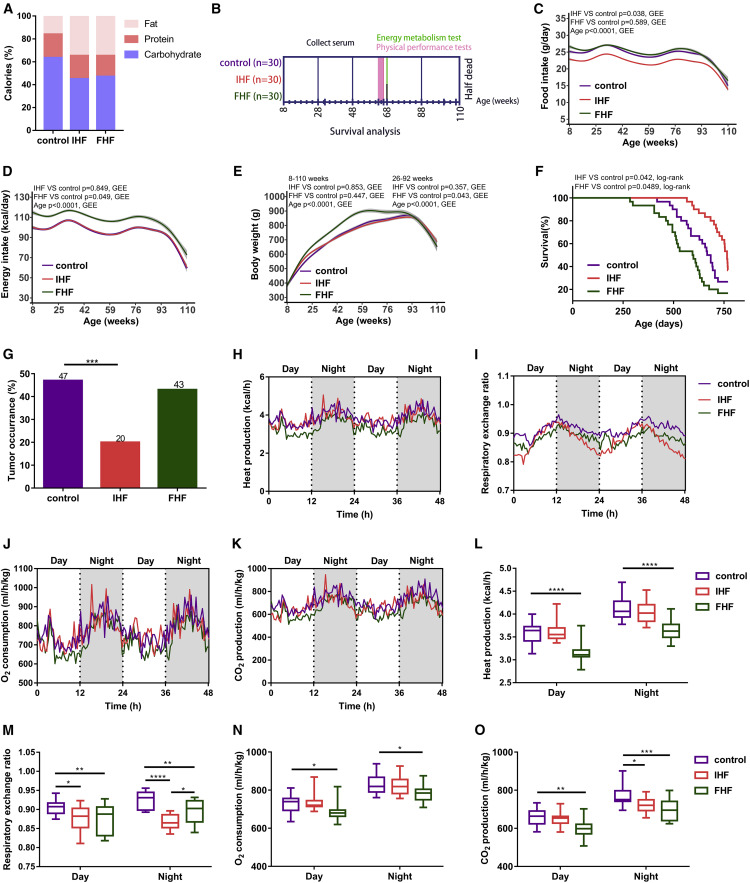
IHF prolongs the lifespan and healthspan of rats

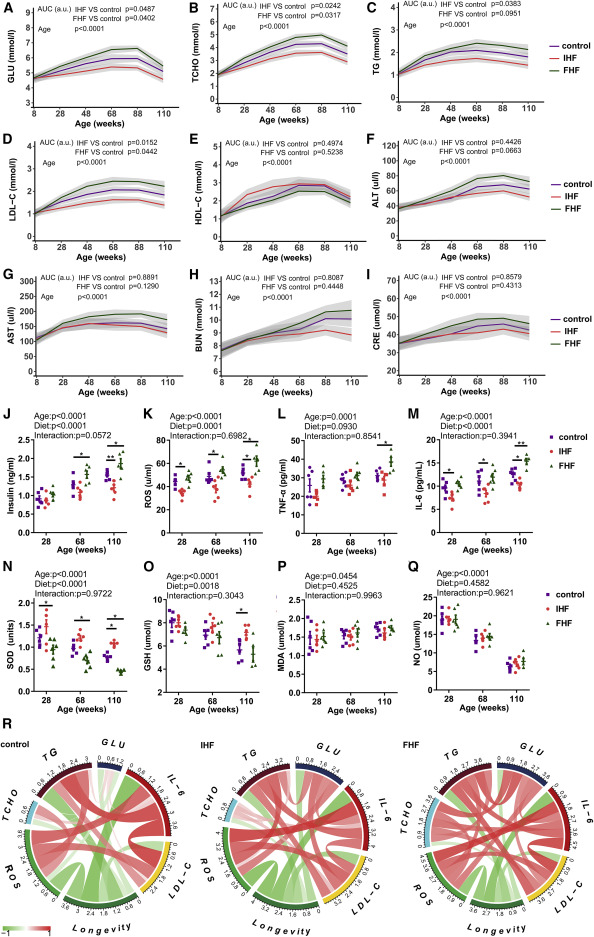
Trajectories of biomarkers of aging during the experiment

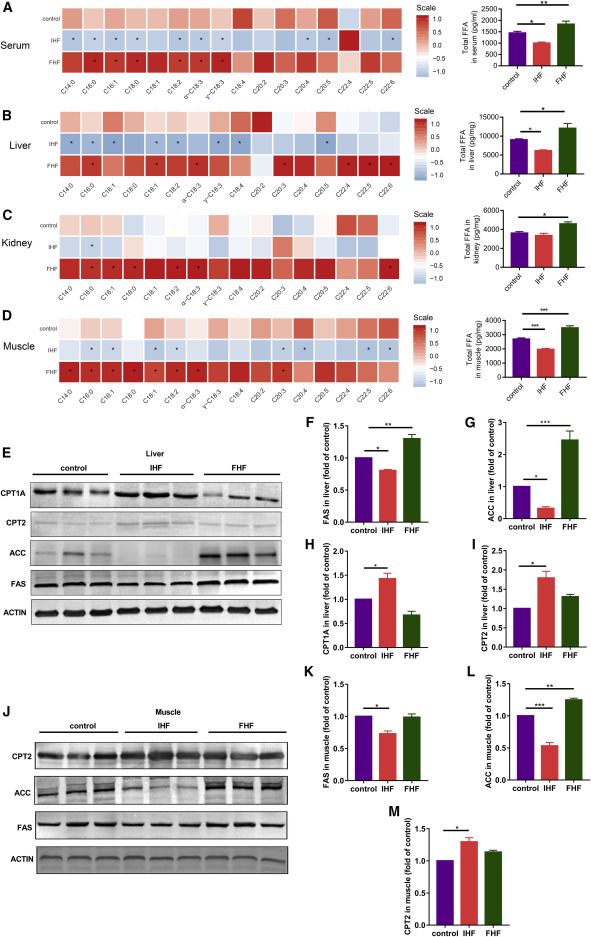
Profiles of fatty acids in serum, liver, kidney, and muscle
- Inoguchi T.
- Li P.
- Umeda F.
- Yu H.Y.
- Kakimoto M.
- Imamura M.
- Aoki T.
- Etoh T.
- Hashimoto T.
- Naruse M.
- et al.

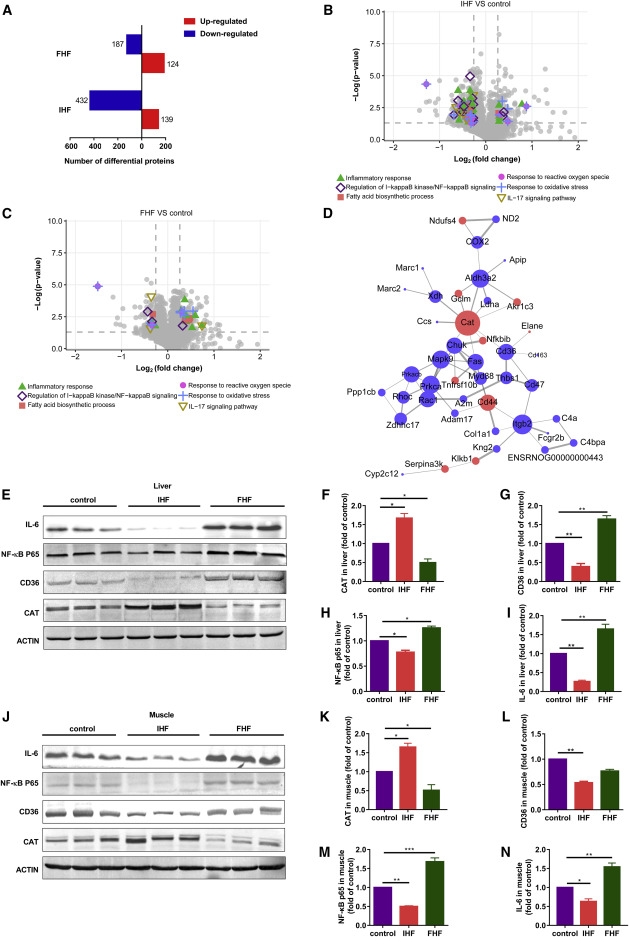
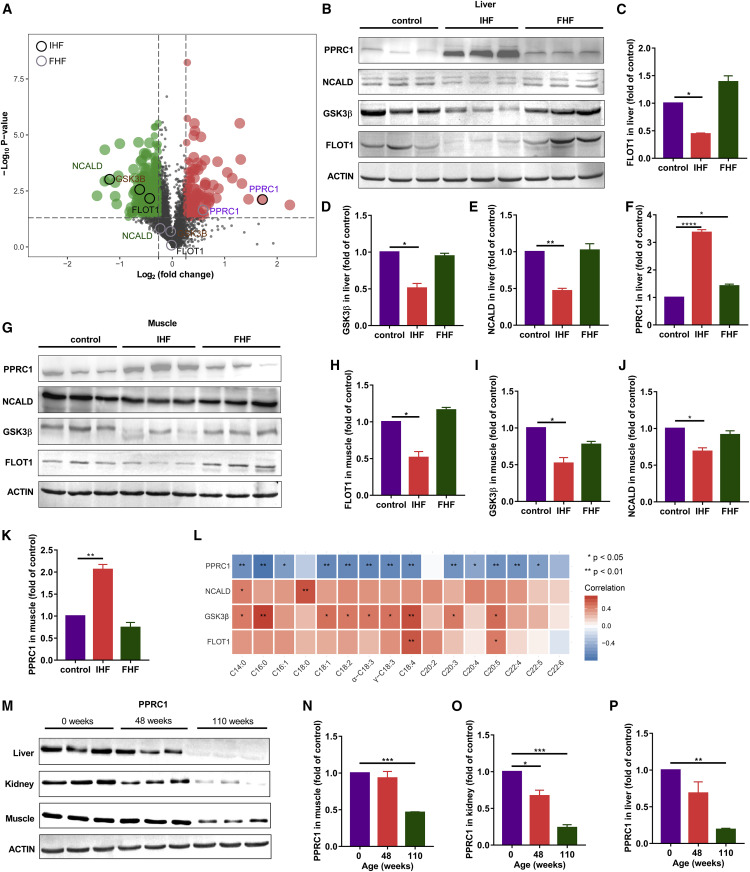
Liver proteomic changes further reflected the above observations and identified PPRC1 as a candidate target

- Sheedy F.J.
- Grebe A.
- Rayner K.J.
- Kalantari P.
- Ramkhelawon B.
- Carpenter S.B.
- Becker C.E.
- Ediriweera H.N.
- Mullick A.E.
- Golenbock D.T.
- et al.

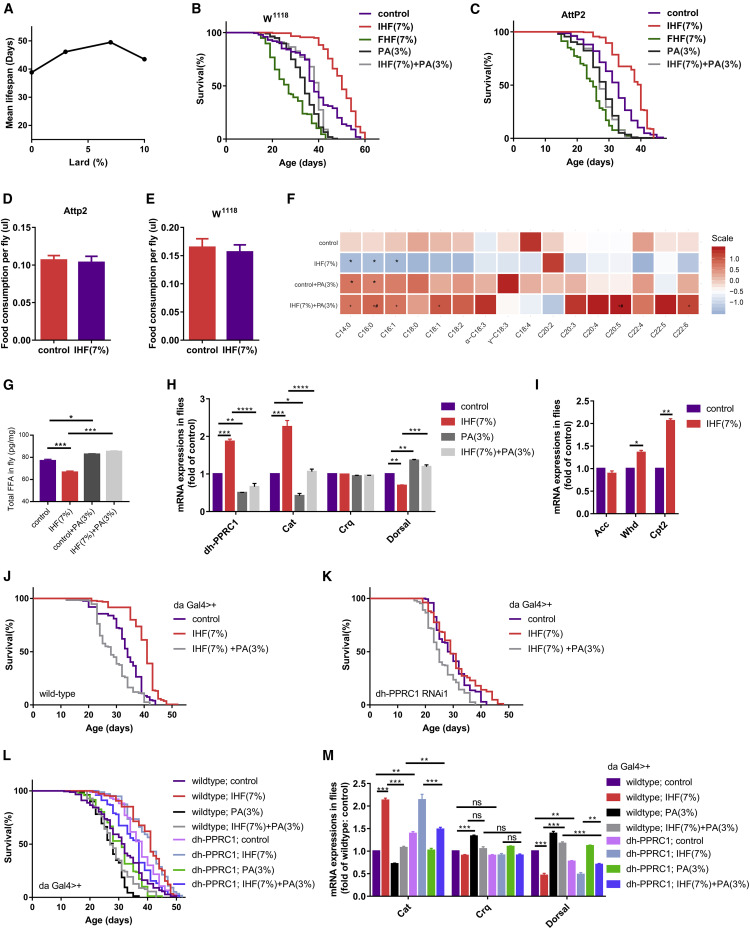
IHF also prolonged the lifespan in flies

Transgenic RNAi and overexpression flies
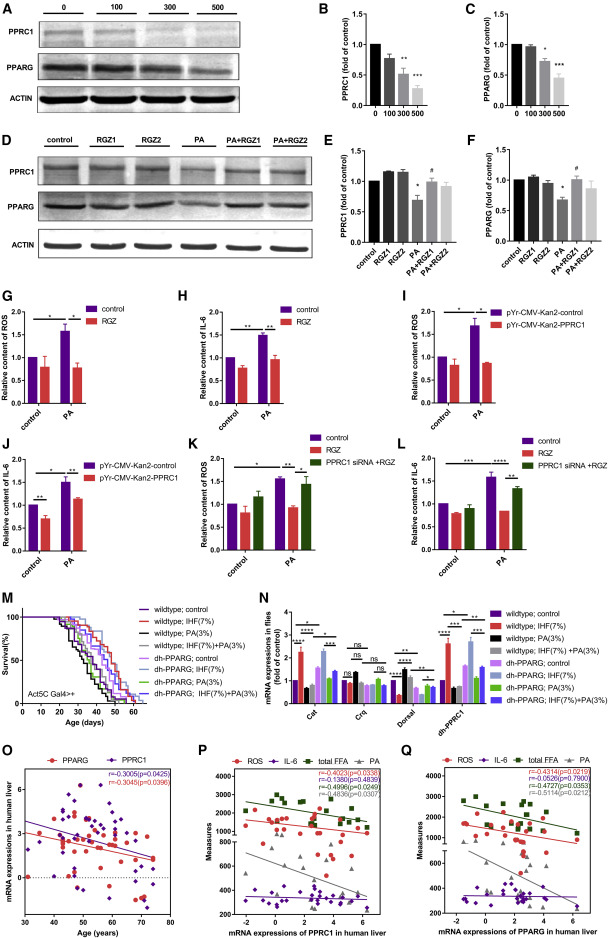
Decreased PA upregulates PPRC1, contributing to IHF-mediated changes in lifespan, oxidative stress, and inflammation through PPARG

PPARG/PPRC1 inversely correlates with age detected by human liver
Beta-hydroxybutyrate is a secondary contributor to the IHF-mediated reduction in oxidative stress and inflammation in rats
Discussion
- Dehghan M.
- Mente A.
- Zhang X.
- Swaminathan S.
- Li W.
- Mohan V.
- Iqbal R.
- Kumar R.
- Wentzel-Viljoen E.
- Rosengren A.
- et al.
Conclusions
Limitations of study
STAR★methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal Anti-Flotillin-1 | Cell Signaling Technology | Cat# 18634; RRID: AB_277304081 |
| Rabbit Anti-Fatty Acid Synthase | Cell Signaling Technology | Cat# 3180; RRID: AB_2100796 |
| Rabbit monoclonal Anti-Catalase | Cell Signaling Technology | Cat# 14097; RRID: AB_2798391 |
| Rabbit monoclonal Anti-GSK-3β | Cell Signaling Technology | Cat# 12456; RRID: AB_26369787 |
| Rabbit polyclonal Anti-Acetyl-CoA Carboxylase | Cell Signaling Technology | Cat# 3676; RRID: AB_2219397 |
| Mouse monoclonal Anti-β-Actin | Cell Signaling Technology | Cat# 58169; RRID: AB_2750839 |
| Rabbit polyclonal Anti-CD36 | Abcam | Cat# ab80978; RRID: AB_1640333 |
| Rabbit polyclonal Anti-NCALD | Absin Bioscience | Abs139207 |
| Mouse polyclonal Anti-CPTII | Santa Cruz Biotechnology | Sc-377294 |
| Rabbit polyclonal Anti-NFκB p65 | WanLeiBio | WL 01273b |
| Anti-IL6 Rabbit pAb | WanLeiBio | WL02841 |
| Mouse monoclonal Anti-CPT1A | Abcam | Cat# ab128568; RRID: AB_11141632 |
| Rabbit monoclonal Anti-PPARγ | Cell Signaling Technology | Cat# 2443; RRID: AB_823598 |
| Rabbit monoclonal Anti-PPARγ | WanLeiBio | WL01800 |
| Mouse monoclonal Anti-PPRC1 | Santa Cruz Biotechnology | Cat# sc-376431; RRID: AB_11149017 |
| Rabbit polyclonal Anti-PPRC1 | OriGene Technologies | Cat# AP53426PU-N; RRID: AB_11145429 |
| Anti-Rabbit IgG (Fc), AP Conjugate | Promega | Cat# S3731; RRID: AB_430872 |
| Anti-Mouse IgG (H+L), AP Conjugate | Promega | Cat# S3721; RRID: AB_430871 |
| Chemicals, peptides, and recombinant proteins | ||
| Control diet (custom) | N/A | See Table S1 |
| IHF diet (custom) | N/A | See Table S1 |
| FHF diet (custom) | N/A | See Table S1 |
| Palmitic acid | Sigma-Aldrich | P5585 |
| Lipofectamine 2000 Reagent | Invitrogen | Cat#11668027 |
| pYr-CMV-Kan2-PPRC1 | Yingrun Biotechnology | HO015062 |
| pYr-CMV-Kan2-control | Yingrun Biotechnology | N/A |
| PPRC1 siRNA | Santa Cruz Biotechnology | Sc-90572 |
| Control siRNA-A | Santa Cruz Biotechnology | Sc-37007 |
| Rosiglitazone | MedChemExpress LLC | CAS#122320-73-4 |
| Pioglitazone | MedChemExpress LLC | CAS#111025-46-8 |
| TRIzol reagent | Invitrogen | Cat#15596026 |
| RIPA lysis buffer | Beyotime | P0013C |
| Phenylmethanesulfonyl fluoride (PMSF) | Beyotime | ST506 |
| Trypsin Gold, Mass Spectrometry Grade | Promega | V5280 |
| Rat ACTB Endogenous Reference Genes Primers | Sangon Biotech | B661202-0001 |
| Human ACTB Endogenous Reference Genes Primers | Sangon Biotech | B661102-0001 |
| Critical commercial assays | ||
| Human IL-6 ELISA | Lengton Bioscience | BPE10140 |
| Human ROS ELISA | Lengton Bioscience | BPE11725 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | 4374966 |
| Total Nitric Oxide Assay Kit | Beyotime | S0024 |
| GSH and GSSG Assay Kit | Beyotime | S0053 |
| Lipid Peroxidation MDA Assay Kit | Beyotime | S0131 |
| Total Superoxide Dismutase Assay | Beyotime | S0101 |
| Mouse/Rat Insulin ELISA Kit | Westang | N/A |
| Mouse/Rat IL-6 ELISA Kit | Biotopped | TOPEL02879 |
| Mouse/Rat TNF-α ELISA Kit | Biotopped | TOPEL02868 |
| Mouse/Rat ROS ELISA Kit | Biocompare | Abx156053 |
| Beta-hydroxybutyrate Assay Kit | Sigma-Aldrich | MAK041 |
| Deposited data | ||
| The proteomics data | This paper | Identifier: PXD022585 |
| Experimental models: cell lines | ||
| Human hepatocytes (HepG2) cells | Chinese Academy of Science | N/A |
| Experimental models: organisms/strains | ||
| Wistar rat | Vital River Laboratory Animal Technology Company LTD | N/A |
| W1118 | Tsinghua Drosophila Stock Center | THJ0265 |
| Dh-PPRC1 RNAi-1 | Tsinghua Drosophila Stock Center | HMS00857 |
| Dh-PPRC1 RNAi-2 | Tsinghua Drosophila Stock Center | HMS00858 |
| Dh-PPRC1 overexpressions using flySAM Transgenic CRISPRa | Tsinghua Drosophila Stock Center | TH14666S |
| UAS-Eip75B overexpressions (genotype: w∗; p {UAS-Eip75B} attP2/ TM6B) | Fungene Biotechnology | N/A |
| y[1] w[∗]; P{w[+mC]=Act5C-GAL4} 25FO1/CyO,y[+] | Fungene Biotechnology | N/A |
| Ubi-Gal4 | Tsinghua Drosophila Stock Center | TB00152 |
| Daughterless Gal4 | Tsinghua Drosophila Stock Center | TB00153 |
| Tub Gal4/TM6b, Tb | Tsinghua Drosophila Stock Center | TB00129 |
| y v; attP2, y+ | Tsinghua Drosophila Stock Center | TB00072 |
| Software and algorithms | ||
| Prism 7.0 | GraphPad | http://www.graphpad.com/scientific-software/prism; RRID: SCR_015807 |
| SPSS 17.0 | SPSS | 17.0 |
| R (version 3.5.1) | R | https://www.r-project.org/ |
| DAVID tool | DAVID | http://david.ncifcrf.gov/ |
| STRING | STRING | https://string-db.org/ |
| Cytoscape (version 3.6.1) | Cytoscape Consortium | https://cytoscape.org/ |
| Thermo Scientific Xcalibur 3.1 | Xcalibur | 3.1 |
| Other | ||
| Shuttle box tests | Taimeng | N/A |
| Morris water maze | Taimeng | N/A |
| Treadmill | Taimeng | N/A |
| ROCHE Modular P800 Biochemical Analyzer | Roche Diagnostics | N/A |
| The indirect calorimetry | TSE | N/A |
| Calibration capillary | VWR, West Chester, PA | #53432-706 |
| GC/MS-MS | Thermo Finnigan | N/A |
Resource availability
Lead contact
Materials availability
Data and code availability
Experimental model and subject details
Animals and diets
| Component (g) | Control | IHF | FHF |
|---|---|---|---|
| Casein | 200.0 | 220.4 | 200.0 |
| L-cystine | 3.0 | 3.5 | 3.0 |
| Cornstarch | 397.5 | 304.5 | 325.5 |
| Dextrinized cornstarch | 132.0 | 114.4 | 114.0 |
| Sucrose | 100.0 | 80.0 | 88.0 |
| Soybean oil | 70.0 | 70.0 | 70.0 |
| Lard | 0.0 | 100.0 | 102.0 |
| Mixed minerals | 35.0 | 38.6 | 35.0 |
| Mixed vitamins | 10.0 | 11.0 | 10.0 |
| Cellulose | 50.0 | 55.1 | 50.0 |
| Choline | 2.5 | 2.5 | 2.5 |
| 1000.0 | 1000.0 | 1000.0 | |
| Energy from macronutrients (%) | |||
| Carbohydrate | 63.6 | 45.1 | 47.2 |
| Protein | 20.5 | 20.3 | 18.2 |
| Fat | 15.9 | 34.6 | 34.6 |
Drosophila stocks
| Component (g) | Larval media | Control (0%) | IHF (3%) | IHF (7%) | IHF (10%) |
|---|---|---|---|---|---|
| Agar | 10 | 11 | 11 | 11 | 11 |
| Dextrose | 55 | 145 | 103.53 | 48.29 | 1.52 |
| Corn meal | 60 | 50 | 50 | 50 | 50 |
| Sucrose | 30 | 91 | 64.97 | 30.3 | 9.56 |
| Yeast | 25 | 18 | 18 | 18 | 18 |
| 20% Tegosept | 15 | 15 | 15 | 15 | 15 |
| Propionic acid | 3 | 3 | 3 | 3 | 3 |
| Lard | 0 | 0 | 30 | 70 | 100 |
| Water | 1000ml | 1000ml | 1000ml | 1000ml | 1000ml |
| Calorie (cal) | |||||
| Carbohydrate | — | 1087.45 | 817.45 | 457.81 | 187.77 |
| Protein | — | 75.46 | 75.46 | 75.46 | 75.46 |
| Fat | — | 15.50 | 285.50 | 645.50 | 915.50 |
| Total calorie | — | 1178.402 | 1178.402 | 1178.762 | 1178.722 |
Cell culture and treatment
Liver sample collection in humans
Method details
Physical performance tests
Collection of blood and tissue
Indirect calorimetry
Proteomics analysis
Protein extraction
- (1)Mix 0.5 ml of lysis buffer 3 (8 M Urea, 40 mM Tris-HCl or TEAB with 1mM PMSF, 2mM EDTA and 10mM DTT, pH 8.5) with 100 mg of liver sample, and place in a 1.5 ml centrifuge tube.
- (2)The mixtures were placed into a TissueLyser with two magnetic beads (diameter 5mm) for 2min at 50Hz to release proteins.
- (3)Centrifugation with 25,000g at 4°C for 20min, and the supernatant was transferred into a new tube.
- (4)Add 10 mM dithiothreitol (DTT) to the supernatant, and 56°C water bath for 1 hour.
- (5)Return to room temperature and then alkylate by 55 mM iodoacetamide (IAM) in the dark at room temperature for 45min.
- (6)Following centrifugation (25,000 g, 4°C, 20 min), the supernatant containing proteins was quantified by Bradford
Quality control of protein extraction
- (1)Protein quantitation with bradford assay. Protein was quantitated according to the manufacturers' protocol.
- (2)SDS-PAGE. After Mixing 30μg proteins with loading buffer in centrifuge tube, heat them at 95°C for 5 minutes to make protein sample. load the protein sample to holes in 12% polyacrylamide gel and then run SDS-PAGE at 120V constant voltage for 120 minutes. After electrophoresis, stain gel with Coomassie Blue for 2 hours, then add destaining solution (40% ethanol and 10% acetic acid) and shake it for 3-5 times, each time for 30 minutes.
Protein digestion
Peptide labeling
Peptide fractionation
HPLC
Mass spectrometer detection
Bioinformatics pipeline
MS/MS raw data
MS/MS ion search and canonical proteomics database
| Item | Value |
|---|---|
| Type of search | MS/MS ion search |
| Enzyme | Trypsin |
| Fragment mass tolerance | 0.05Da |
| Mass values | Monoisotopic |
| Variable modifications | Oxidation (M), iTRAQ8plex (Y) |
| Peptide mass Tolerance | 20ppm |
| Fixed modifications | Carbamidomethyl (C), iTRAQ8plex (N-term), iTRAQ8plex (K) |
| Database | I-cxPBT002 (35814 sequences) |
| Database_info | Rtus_norvegicus(uniprot,20171117) |
Protein quantification
| Item | Value |
|---|---|
| Quant_peptide | Use All Unique peptide |
| Quant_number | At least one unique spetra |
| Normalization | VSN |
| Protein_Ratio | Weighted average |
| Statistical Analysis | Permutation Tests |
GO enrichment and pathway enrichment
Western blot analysis
Quantitative real-time PCR
| Primers | Sequences |
|---|---|
| fruit fly-rpl32-FORWARD | GCACTTCATCCGCCACCAGTC |
| fruit fly-rpl32-REVERSE | TGCGCTTGTTCGATCCGTAACC |
| fruit fly-dh-PPRC1(spargel)-FORWARD | AAGGAAGCACCAGCACCGAATG |
| fruit fly-dh-PPRC1(spargel)-REVERSE | GTGCCTCCAGCGTAGATGAACC |
| fruit fly-dh-PPARG(Eip75B)-FORWARD | CTCCACCATCAGCATCAGCATCAG |
| fruit fly-dh-PPARG(Eip75B)-REVERSE | CGCCGACTCCAGCAACTTGAC |
| fruit fly-whd-FORWARD | TGTTCGACCGCTGCTTGATGATG |
| fruit fly-whd-REVERSE | ACTCCTCCCACCAATCCGACAC |
| fruit fly-crq-FORWARD | TGCTGAACCATGAAGGCGGAAAG |
| fruit fly-crq-REVERSE | GCCACCCGAAGCGTTTGTAGG |
| fruit fly-cpt2-FORWARD | GCCGATGGGAAGGTGTTCATAGAC |
| fruit fly-cpt2-REVERSE | GCTACTGCTGCTCTGCTGGATG |
| fruit fly-acc-FORWARD | AGCAGGCAGGTCAGGTGTGG |
| fruit fly-acc-REVERSE | CAATCAGCGGCAACTCCTCTCG |
| fruit fly-dorsal-FORWARD | AGCAGCAGCAGCAGCAACAG |
| fruit fly-dorsal-REVERSE | TGCCGCCGAGAATTTACTTTCCC |
| fruit fly-cat-FORWARD | GAACGGCTATGGCTCGCACAC |
| fruit fly-cat-REVERSE | CCAACTGATCGGCGGTCTTCAC |
| human-actb- FORWARD | CCTGGCACCCAGCACAAT |
| human-actb- REVERSE | GGGCCGGACTCGTCATAC |
| human-pparg-FORWARD | AGATCATTTACACAATGCTGGC |
| human-pparg-REVERSE | TAAAGTCACCAAAAGGCTTTCG |
| human-pprc1-FORWARD | AACTACGGCTTCGTCACTTATC |
| human-pprc1-REVERSE | AGATCAGAATAGCTCCTCTTGC |
| rat-actb-FORWARD | TGTCACCAACTGGGACGATA |
| rat-actb- REVERSE | GGGGTGTTGAAGGTCTCAAA |
| rat-ncald-FORWARD | GCCAGATGGACACCAACAGAGATG |
| rat-ncald-REVERSE | GCAGGAGACGCACAATGGATGG |
| rat-flot1-FORWARD | TGGACATGCTGCTGGAGAAACTG |
| rat-flot1-REVERSE | CATGGTTCCGCTTCCGCTAGAC |
| rat-gsk3b-FORWARD | CAATCGCACTGTGTAGCCGTCTC |
| rat-gsk3b-REVERSE | GGTGTGTCTCGCCCATTTGGTAG |
| rat-pprc1-FORWARD | GCTGATGATCTGACACTGCCTGAG |
| rat-pprc1-REVERSE | GAATCTGCCGCACGACCACTG |
| rat-scot-FORWARD | GGGGTGTGCCTGCTACTTTTCC |
| rat-scot-REVERSE | ACACAACCCGAAACCACCAACC |
Food intake measurement in Drosophila
Quantification and statistical analysis
Survival assessment
Pathology examinations and quantification
Blood chemistry measurement
Free fatty acids measurement
Blood beta-hydroxybutyrate levels
Statistical analysis
Acknowledgments
Author contributions
Declaration of interests
Supplemental information
- Document S1. Figures S1–S7 and Tables S1–S4
- Data S1. Original datasets of the results in this manuscript
(A) Pathological records of rats related to Figures 1 and S1.
(B) Survival data of rats related to Figure 1F.
(C) Energy metabolism of rats related to Figures 1H−1O.
(D) Differential protein expressions with regard to oxidative stress and inflammation in the IHF-control/FHF pairwise comparisons related to Figures 4B and 4C
(E) protein-protein interaction differential protein expressions with regard to oxidative stress and inflammation in the IHF-control pairwise comparisons related to Figures 4D and 4F Differential protein expressions with regard to nutrient-sensing and fatty acids metabolism in the IHF-control pairwise comparisons related to Figures S3 and S4.
(G) All proteins in the IHF-control pairwise comparisons related to Figures S3–S5.
(H) All proteins in the FHF-control pairwise comparisons related to Figures S3–S5.
(I) protein-protein interaction for all differential proteins related to Figure S3E.
- Data S2. Proteins and pathways regulated by IHF or FHF versus control, related to Figures 4B, 4C, S3A, and S3B
- Data S3. Statistical analyses of survival curves for flies, related to Figures 6, 7, and S5
References
- CD36 actions in the heart: lipids, calcium, inflammation, repair and more?.Biochim. Biophys. Acta. 2016; 1861: 1442-1449
- Prolonging healthy aging: longevity vitamins and proteins.Proc. Natl. Acad. Sci. USA. 2018; 115: 10836-10844
- PPARgamma2 is a key driver of longevity in the mouse.PLoS Genet. 2009; 5: e1000752
- High-fat diet enhances stemness and tumorigenicity of intestinal progenitors.Nature. 2016; 531: 53-58
- Free fatty acid and glucose metabolism in human aging: evidence for operation of the Randle cycle.Am. J. Physiol. 1994; 266: E501-E509
- The toxic influence of paraquat on hippocampus of mice: involvement of oxidative stress.Neurotoxicology. 2010; 31: 310-316
- Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease.Nature. 2018; 560: 185-191
- Fat, calories, and cancer.Cancer Res. 2016; 76: 509-510
- Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study.Lancet. 2017; 390: 2050-2062
- The ProteomeXchange Consortium in 2020: enabling 'big data' approaches in proteomics.Nucleic Acids Res. 2020; 48: D1145-D1152
- The capillary feeder assay measures food intake in Drosophila melanogaster.J. Vis. Exp. 2017; 121: 55024
- The functions of PPARs in aging and longevity.PPAR Res. 2007; 2007: 39654
- Reprogramming of energy metabolism as a driver of aging.Oncotarget. 2016; 7: 15410-15420
- Promoting health and longevity through diet: from model organisms to humans.Cell. 2015; 161: 106-118
- Extending healthy life span--from yeast to humans.Science. 2010; 328: 321-326
- Saturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism aging.Age (Dordr.). 2010; 32: 231-237
- Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans.PLoS Biol. 2011; 9: e1000599
- Regulatory T cells expressing PPAR-gamma control inflammation in obesity.Cell Metab. 2012; 16: 4-6
- Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan.Nature. 2017; 544: 185-190
- Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet.Cell Metab. 2012; 15: 848-860
- Reduced expression of MYC increases longevity and enhances healthspan.Cell. 2015; 160: 477-488
- Vitamin D deficiency: a worldwide problem with health consequences.Am. J. Clin. Nutr. 2008; 87: 1080S-1086S
- High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells.Diabetes. 2000; 49: 1939-1945
- The effects of ovariectomy and lifelong high-fat diet consumption on body weight, appetite, and lifespan in female rats.Horm. Behav. 2018; 97: 25-30
- The role of lipid metabolism in aging, lifespan regulation, and age-related disease.Aging Cell. 2019; 18: e13048
- Myristoylation of Src kinase mediates Src-induced and high-fat diet-accelerated prostate tumor progression in mice.J. Biol. Chem. 2017; 292: 18422-18433
- Effects of high-fat diet on incidence of spontaneous tumors in Wistar rats.Nutr. Cancer. 1993; 19: 99-110
- High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program.Nat. Commun. 2019; 10: 4358
- SREBP and MDT-15 protect C. elegans from glucose-induced accelerated aging by preventing accumulation of saturated fat.Genes Dev. 2015; 29: 2490-2503
- The effect of caffeine and albuterol on body composition and metabolic rate.Obesity (Silver Spring). 2015; 23: 1830-1835
- Targeted metabolomics analysis reveals the association between maternal folic acid supplementation and fatty acids and amino acids profiles in rat pups.J. Chromatogr. B. 2018; 1090: 101-109
- Interventions to slow aging in humans: are we ready?.Aging Cell. 2015; 14: 497-510
- The hallmarks of aging.Cell. 2013; 153: 1194-1217
- Mechanisms preserving insulin action during high dietary fat intake.Cell Metab. 2019; 29: 50-63.e54
- Vitamin D supplements and prevention of cancer and cardiovascular disease.N. Engl. J. Med. 2019; 380: 33-44
- Metformin improves healthspan and lifespan in mice.Nat. Commun. 2013; 4: 2192
- Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study.Nature. 2012; 489: 318-321
- Impact of intermittent fasting on health and disease processes.Ageing Res. Rev. 2017; 39: 46-58
- Protein and amino acid restriction, aging and disease: from yeast to humans.Trends Endocrinol. Metab. 2014; 25: 558-566
- Effects of sex, strain, and energy intake on hallmarks of aging in mice.Cell Metab. 2016; 23: 1093-1112
- The 2015 US dietary guidelines: lifting the ban on total dietary fat.JAMA. 2015; 313: 2421-2422
- Ketogenic diet reduces midlife mortality and improves memory in aging mice.Cell Metab. 2017; 26: 547-557.e8
- The phytochemical, EGCG, extends lifespan by reducing liver and kidney function damage and improving age-associated inflammation and oxidative stress in healthy rats.Aging Cell. 2013; 12: 1041-1049
- omega-6 polyunsaturated fatty acids extend life span through the activation of autophagy.Genes Dev. 2013; 27: 429-440
- Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism.Nature. 2017; 543: 252-256
- Linking lipid metabolism to chromatin regulation in aging.Trends Cell Biol. 2019; 29: 97-116
- FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress.Am. J. Physiol. Endocrinol. Metab. 2014; 307: E34-E46
- The PRIDE database and related tools and resources in 2019: improving support for quantification data.Nucleic Acids Res. 2019; 47: D442-D450
- Diet and aging.Cell Metab. 2008; 8: 99-104
- The omega-3 fatty acid alpha-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARalpha and oxidation to oxylipins.Aging Cell. 2017; 16: 1125-1135
- Germline signals deploy NHR-49 to modulate fatty-acid beta-oxidation and desaturation in somatic tissues of C. elegans.PLoS Genet. 2014; 10: e1004829
- Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog.Cell Metab. 2011; 14: 623-634
- A ketogenic diet extends longevity and healthspan in adult mice.Cell Metab. 2018; 27: 1156
- Extension of mouse lifespan by overexpression of catalase.Age (Dordr.). 2006; 28: 209-218
- CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation.Nat. Immunol. 2013; 14: 812-820
- Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor.Science. 2013; 339: 211-214
- Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants.Aging. 2011; 3: 125-147
- A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice.Exp. Mol. Pathol. 2011; 91: 419-428
- Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease.Am. J. Clin. Nutr. 2010; 91: 535-546
- The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-Fed Mice.Cell Metab. 2014; 31: 654
- Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects.Diabetes. 2003; 52: 2882-2887
- Difference of NPY and its receptor gene expressions between obesity and obesity-resistant rats in response to high-fat diet.Horm. Metab. Res. 2007; 39: 262-267
- Fat metabolism links germline stem cells and longevity in C. elegans.Science. 2008; 322: 957-960
- IQuant: an automated pipeline for quantitative proteomics based upon isobaric tags.Proteomics. 2014; 14: 2280-2285
- Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes.Nat. Rev. Cardiol. 2019; 16: 581-601
- Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice.Nat. Commun. 2017; 8: 155
- The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease.Nat. Med. 2015; 21: 263-269
- PPAR-gamma and aging: one link through klotho?.Kidney Int. 2008; 74: 702-704
Article info
Publication history
Identification
Copyright
User license
Elsevier user license |
Permitted
For non-commercial purposes:
- Read, print & download
- Text & data mine
- Translate the article
Not Permitted
- Reuse portions or extracts from the article in other works
- Redistribute or republish the final article
- Sell or re-use for commercial purposes
Elsevier's open access license policy
ScienceDirect
Access this article on ScienceDirectFigures
![Figure thumbnail fx1]() Graphical Abstract
Graphical Abstract![Figure thumbnail gr1]() Figure 1IHF prolongs lifespan and delays tumor occurrence and effects on energy metabolic profiles in rats
Figure 1IHF prolongs lifespan and delays tumor occurrence and effects on energy metabolic profiles in rats![Figure thumbnail gr2]() Figure 2IHF effects on blood biochemistry variables of glucose, lipids, liver/kidney function, oxidative stress, and inflammation
Figure 2IHF effects on blood biochemistry variables of glucose, lipids, liver/kidney function, oxidative stress, and inflammation![Figure thumbnail gr3]() Figure 3IHF effects on free fatty acid profiles in serum, liver, kidney, and muscle
Figure 3IHF effects on free fatty acid profiles in serum, liver, kidney, and muscle![Figure thumbnail gr4]() Figure 4Liver proteomic changes further reflect the improvement of oxidative stress, inflammation, and total FFA metabolism upon IHF
Figure 4Liver proteomic changes further reflect the improvement of oxidative stress, inflammation, and total FFA metabolism upon IHF![Figure thumbnail gr5]() Figure 5Liver proteomic changes further identified PPRC1 as a candidate target
Figure 5Liver proteomic changes further identified PPRC1 as a candidate target![Figure thumbnail gr6]() Figure 6Decreased PA upregulates PPRC1 involved in IHF-mediated change in lifespan, oxidative stress, and inflammation
Figure 6Decreased PA upregulates PPRC1 involved in IHF-mediated change in lifespan, oxidative stress, and inflammation![Figure thumbnail gr7]() Figure 7Decreased PA upregulates PPRC1, contributing to IHF-dependent changes in lifespan, oxidative stress, and inflammation through PPARG
Figure 7Decreased PA upregulates PPRC1, contributing to IHF-dependent changes in lifespan, oxidative stress, and inflammation through PPARG