- Journal List
- EBioMedicine
- v.36; 2018 Oct
- PMC6197652

Fisetin is a senotherapeutic that extends health and lifespan
Matthew J. Yousefzadeh
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Yi Zhu
bRobert and Arlene Kogod Center on Aging, Mayo Clinic, 200 First St., S.W., Rochester, MN 55905, United States
Sara J. McGowan
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Luise Angelini
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Heike Fuhrmann-Stroissnigg
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Ming Xu
bRobert and Arlene Kogod Center on Aging, Mayo Clinic, 200 First St., S.W., Rochester, MN 55905, United States
Yuan Yuan Ling
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Kendra I. Melos
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Tamar Pirtskhalava
bRobert and Arlene Kogod Center on Aging, Mayo Clinic, 200 First St., S.W., Rochester, MN 55905, United States
Christina L. Inman
bRobert and Arlene Kogod Center on Aging, Mayo Clinic, 200 First St., S.W., Rochester, MN 55905, United States
Collin McGuckian
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Erin A. Wade
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Jonathon I. Kato
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Diego Grassi
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Mark Wentworth
cOffice of Research Regulatory Support, Mayo Clinic, Rochester, MN 55905, United States
Christin E. Burd
dDepartment of Molecular Genetics and Cancer Biology and Genetics, The Ohio State University, Columbus, OH 43210, United States
Edgar A. Arriaga
eDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455-0431, United States
Warren L. Ladiges
fDepartment of Comparative Medicine, University of Washington, Seattle, WA 98195, United States
Tamara Tchkonia
bRobert and Arlene Kogod Center on Aging, Mayo Clinic, 200 First St., S.W., Rochester, MN 55905, United States
James L. Kirkland
bRobert and Arlene Kogod Center on Aging, Mayo Clinic, 200 First St., S.W., Rochester, MN 55905, United States
Paul D. Robbins
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Laura J. Niedernhofer
aDepartment of Molecular Medicine and the Center on Aging, The Scripps Research Institute, 130 Scripps Way, Jupiter, FL 33458, United States
Associated Data
Abstract
Background
Senescence is a tumor suppressor mechanism activated in stressed cells to prevent replication of damaged DNA. Senescent cells have been demonstrated to play a causal role in driving aging and age-related diseases using genetic and pharmacologic approaches. We previously demonstrated that the combination of dasatinib and the flavonoid quercetin is a potent senolytic improving numerous age-related conditions including frailty, osteoporosis and cardiovascular disease. The goal of this study was to identify flavonoids with more potent senolytic activity.
Methods
A panel of flavonoid polyphenols was screened for senolytic activity using senescent murine and human fibroblasts, driven by oxidative and genotoxic stress, respectively. The top senotherapeutic flavonoid was tested in mice modeling a progeroid syndrome carrying a p16INK4a-luciferase reporter and aged wild-type mice to determine the effects of fisetin on senescence markers, age-related histopathology, disease markers, health span and lifespan. Human adipose tissue explants were used to determine if results translated.
Findings
Of the 10 flavonoids tested, fisetin was the most potent senolytic. Acute or intermittent treatment of progeroid and old mice with fisetin reduced senescence markers in multiple tissues, consistent with a hit-and-run senolytic mechanism. Fisetin reduced senescence in a subset of cells in murine and human adipose tissue, demonstrating cell-type specificity. Administration of fisetin to wild-type mice late in life restored tissue homeostasis, reduced age-related pathology, and extended median and maximum lifespan.
Interpretation
The natural product fisetin has senotherapeutic activity in mice and in human tissues. Late life intervention was sufficient to yield a potent health benefit. These characteristics suggest the feasibility to translation to human clinical studies.
Fund
NIH grants P01 AG043376 (PDR, LJN), U19 {"type":"entrez-nucleotide","attrs":{"text":"AG056278","term_id":"16593737"}}AG056278 (PDR, LJN, WLL), R24 AG047115 (WLL), R37 AG013925 (JLK), R21 AG047984 (JLK), P30 DK050456 (Adipocyte Subcore, JLK), a Glenn Foundation/American Federation for Aging Research (AFAR) BIG Award (JLK), Glenn/AFAR (LJN, CEB), the Ted Nash Long Life and Noaber Foundations (JLK), the Connor Group (JLK), Robert J. and Theresa W. Ryan (JLK), and a Minnesota Partnership Grant (AMAY-UMN#99)-P004610401–1 (JLK, EAA).
Research in Context
Evidence before this study
Pharmacological targeting of fundamental mechanisms of aging has the ability to reduce the severity or delay the onset of multiple age-associated co-morbidities simultaneously. One key mechanism demonstrated to drive aging is cellular senescence, whereby accumulation of DNA damage and/or other cellular stressors cause proliferating or terminally differentiated non-dividing cells to enter a state characterized by profound chromatin and secretome changes, increased expression of the cell cycle inhibitor p16Ink4a in many but not all senescent cells, replicative arrest, and resistance to apoptosis. Senescent cells can develop a senescence-associated secretory phenotype (SASP), which has deleterious paracrine and systemic effects. Senescent cells are rare in young individuals, but increase with age in multiple tissues. Drugs able to selectively kill senescent cells, termed senolytics, have been identified including the combination of dastinib and quercetin (D ± Q), which improves many aspects of aging in mouse models of accelerated and natural aging. However, safer and improved drugs targeting senescence likely are needed to eliminate senescent cells safely from multiple organs or even within a single tissue.
Added value of the study
This study identifies the flavonoid polyphenol fisetin as having greater senotherapeutic activity in cultured cells than quercetin. In addition, fisetin had potent senotherapeutic activity in vivo. Treatment of progeroid and aged wild-type mice acutely or intermittently with fisetin reduced senescence markers in multiple tissues and a subset of cell types in adipose tissue. Importantly, chronic administration of fisetin to wild-type mice late in life improved tissue homeostasis, suppressed age-related pathology, and extended median and maximum lifespan. This result, similar to a recent report on the combination of D ± Q, is the first to document extension of both health span and lifespan by a senolytic with few side effects, even though administration was started late in life.
Implications of all the available evidence
Taken together, these data establish the natural product fisetin as a potent senotherapeutic, able to reduce the burden of senescent T, NK, progenitor, and endothelial cells from fat tissue, and demonstrate that reducing the senescent cell burden in mice even late in life is sufficient to have a significant health impact. Given the known safety profile of fisetin in humans, clinical trials are beginning in order to test if fisetin can be used effectively to reduce senescent cell burden and alleviate dysfunction in elderly subjects.
Alt-text: Unlabelled Box
1. Introduction
Pharmacologically targeting fundamental mechanisms of aging is anticipated to reduce the severity or delay the onset of multiple age-associated co-morbidities simultaneously [[5], [6], [7]]. One key mechanism demonstrated to drive aging is cellular senescence, whereby accumulation of DNA damage and/or other cellular stressors [[1], [2], [3], [4]] cause proliferating [8,9] or terminally differentiated non-dividing cells [[10], [11], [12], [13]] to enter a state characterized by profound chromatin and secretome changes, increased expression of the cell cycle inhibitor p16Ink4a, replicative arrest, and resistance to apoptosis [1,14]. Senescent cells can develop a senescence-associated secretory phenotype (SASP), consisting of pro-inflammatory cytokines, chemokines, and extracellular matrix-degrading proteins [[15], [16], [17], [18]], which has deleterious paracrine and systemic effects [[19], [20], [21]]. Indeed, even a relatively low abundance of senescent cells is sufficient to cause tissue dysfunction [22]. Senescent cells are rare in young individuals, but increase with age in multiple tissues, including adipose tissue, skeletal muscle, kidney, and skin of all vertebrates tested [22,23].
The role of senescent cells in age-related decline was identified by studies demonstrating the therapeutic benefits of clearing of senescent cells from progeroid or naturally-aging INK-ATTAC mice using a suicide gene expressed only in p16Ink4a expressing cells (J.L.K., T.T., J.M. van Deursen, and D.J. Baker [all Mayo Clinic] designed the INK-ATTAC strategy [19,20,[24], [25], [26]]). Conversely, injection of senescent cells is sufficient to drive age-related conditions such as osteoarthritis, frailty, and decreased survival [26,27]. Thus, the development of therapies that selectively kill senescent cells was anticipated to delay the onset of aging phenotypes, attenuate severity of age-related diseases, improve resiliency, and enhance survival. Importantly, it was also predicted that senolytic therapies could be administered intermittently, serving to reduce the senescent cell burden by treating quarterly or even annually, which minimizes the risk of side effects [28,29].
We previously identified drugs that selectively kill senescent cells using a hypothesis-driven discovery paradigm [30]. Senescent cells are resistant to apoptosis due to upregulation of Senescent-Cell Anti-Apoptotic Pathways (SCAPs) [28,29]. Targeting SCAPs in cell culture using a combination of dasatinib and quercetin, an inhibitor of BCL-2 pro-survival pathway members, Navitoclax, or the more specific BCL-xL inhibitor, A1331852, results in apoptosis of some but not all senescent cell types [[30], [31], [32], [33]]. Treatment of mice with dasatinib plus quercetin (D + Q) improves cardiac ejection fraction and increases vascular reactivity in old mice after a single, 3 day treatment course [30,34]. In addition, D + Q treatment decreases vascular calcification and increases vascular reactivity in hypercholesterolemic, high fat diet fed ApoE−/− mice after three monthly 3 day treatment courses [34]. Intermittent oral D + Q treatment improves pulmonary function and reduces pulmonary fibrosis in a bleomycin-induced murine model of idiopathic pulmonary fibrosis [35], reduces high fat diet-induced liver steatosis [36], alleviates gait impairment caused by leg irradiation [30] and reduces osteoporosis in aged mice [10]. Finally, D + Q also decreases frailty, osteoporosis, loss of intervertebral disc glycosaminoglycans, and spondylosis in the Ercc1−/Δ mouse model of a human progeroid syndrome after intermittent treatment [30]. Similarly, Navitoclax, which decreases abundance of some but not all human and mouse senescent cell types in vitro [33], reduces hematologic dysfunction caused by whole body radiation [31] and reduces senescent cell-like, intimal foam cell/macrophages in vascular plaques in high fat fed LdlR−/− mice [37]. Treatment with A1331852 reduces senescent cholangiocytes and liver fibrosis in Mdr2−/− mice [38]. Taken together, these studies demonstrate that senolytic compounds can have significant effects on chronic degenerative diseases and age-related pathology.
However, not all senescent cells are the same. Senescent cells may express different SASP factors, senescence markers, and more importantly use different mechanisms to resist apoptosis [30,39]. Furthermore, certain cancer therapeutics target SCAPs, e.g. Navitoclax, and could be repurposed as senolytics, but cause considerable toxicity including neutropenia and platelet deficiency [40,41]. Thus, new and improved senotherapeutic drugs and combinatorial approaches are needed to eliminate senescent cells safely from multiple organs or even within a single tissue [[28], [29], [30],42].
Here, we screened a panel of flavonoids for senotherapeutic activity to determine if we could improve upon quercetin. In primary murine embryonic fibroblasts induced to senescence through oxidative stress and in human fibroblasts induced to senescence with the genotoxin etoposide, fisetin was most effective at reducing senescent markers. Fisetin also reduced senescence markers in progeroid Ercc1−/∆ mice and aged WT mice, as well as human explants of adipose tissue. Fisetin treatment extended the health and lifespan in WT mice even when treatment was initiated in aged animals. This flavonoid is a natural compound present in many fruits and vegetables such as apples, persimmon, grapes, onions, cucumbers and strawberries [43,44], suggesting that it is imminently translatable. Importantly, no adverse effects of fisetin have been reported, even when given at high doses [45]. Thus, our results suggest that supplementation or even intermittent treatment with this safe, natural product could improve healthy aging, even in elderly individuals.
2. Materials and methods
2.1. Chemicals
Chemicals were from Sigma-Aldrich (St. Louis) unless otherwise noted. The flavonoids were purchased from Selleckchem (Houston, TX): resveratrol (Cat #S1396), fisetin (Cat #S2298), luteolin (Cat #S2320), rutin (Cat #S2350), epigallocatechin gallate (EGCG, Cat #S2250), curcumin (Cat #S1848), pirfenidone (Cat #S2907), and myricetin (Cat #S2326). Apigenin, catechin, and quercetin were purchased from Sigma-Aldrich (Cat #1760595, #1096790 and 1,592,409, respectively).
2.2. Animals
All animal studies were conducted in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and were approved by the Scripps Florida or Mayo Clinic Institutional Animal Care and Use Committees. Ercc1−/∆ mice were bred as previously described [46]. p16-luciferase reporter mice were obtained from Ohio State University [47] and bred to create an albino C57BL/6 p16Luc/+;Ercc1+/− and FVB/n p16+/Luc;Ercc1+/∆ strain. These mice were further crossed to create f1 p16+/Luc;Ercc1−/Δ mice with white fur for imaging. All animals were genotyped from an ear punch by TransnetYX (Cordova, TN). For diet studies, mice were fed Teklad 2020 chow (Envigo, Madison, WI) prepared with or without 500 ppm (500 mg/kg) of fisetin (Indofine Chemical Co., Hillsborough, NJ) by Envigo. Co. (Tampa, FL). For oral administration of fisetin, mice were dosed with 100 mg/kg of fisetin in 60% Phosal 50 PG:30% PEG400:10% ethanol or vehicle only by gavage. Studies in aged wild-type mice were conducted in both f1 C57BL/6;FVB/n and inbred C57BL/6 genetic backgrounds.
2.3. MEF isolation
The Ercc1−/− MEFs were isolated from pregnant females at embryonic day 13 (E13) and cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F10 with 10% fetal bovine serum, 1× nonessential amino acids, penicillin, and streptomycin and incubated at 3% O2 initially, followed by a shift to 20% for 5 passages to induce senescence [48]. Cells were genotyped by TransnetYX (Cordova, TN) and routinely tested for mycoplasma contamination using the MycoAlert PLUS mycoplasma detection kit (Lonza, Walkersville, MD).
2.4. Assays to identify senotherapeutics
Ercc1−/− MEFs were passaged 5 times at 20% O2 to induce senescence then seeded at 5000 cells per well in 96 well plates at least 6 h prior to treatment. Following the addition of drugs, the MEFs were incubated for 24–48 h at 20% O2. Subsequently SA-β-gal activity was measured in three independent experiments, as previously described [49]. Briefly, cells were washed with PBS then 10 μM C12FDG added in fresh culture medium and incubated for 2 h. Ten min prior to analysis, 2 μg/mL Hoechst dye was added. An IN Cell Analyzer 6000 was used to quantitate total number of viable cells (Hoechst+) and the number of senescent cells (C12FDG+). All samples were analyzed in duplicate with 3–5 fields per well and reported as the mean ± S.D. Senotherapeutic activity was confirmed in human fibroblasts (IMR90). The cells were obtained from American Type Culture Collection (ATCC) and cultured in EMEM medium with 10% FBS and antibiotics. To induce senescence, the cells were treated for 24 h with 20 μM etoposide. Two days after etoposide removal, ~70% of the cells were SA-β-gal+. Cells were treated for 48 h with different concentrations of fisetin (1–15 μM) and the percentage of SA-β-gal+ cells was determined using C12FDG, as described above.
2.5. IVIS in vivo imaging detection of luciferase activity
Isoflurane-anesthetized mice (n = 2–10 mice per group) were injected intraperitoneally with 10 μL per gram body weight D-luciferin substrate (Caliper Life Sciences, Hopkinton, MA; 15 mg/mL diluted in sterile PBS) and were imaged using an IVIS Lumina (PerkinElmer, Billerica, MA), as previously described [47,50].
2.6. Measurement of lipid peroxidation
Levels of 4-hydroxynonenal-protein adducts of liver lysates (n = 4–6 mice per group) prepared in RIPA buffer were measured in the livers of mice using the OxiSelect HNE Adduct Competitive ELISA kit (Cell Biolabs, San Diego, CA), as described [50].
2.7. Measurement of glutathione
Murine livers (n = 4–7 mice per group) fixed in 5% sulfosalicylic acid were prepared and analyzed for the concentration of reduced (GSH) and oxidized (GSSG) glutathione using the Glutathione Assay Kit (Cayman Chemical, Ann Arbor, MI), as described [50]. Sample absorbance was measured at 405 nm using a plate reader and the ratio of GSH:GSSG was reported for each sample.
2.8. Clinical chemistries
Whole blood (n = 3–6 mice per group) was collected immediately following animal euthanasia via cardiac puncture into heparinized tubes for analysis of clinical chemistries utilizing VetScan Comprehensive Diagnostic Profile rotors on a VestScan VS2 (Abaxis, Union City, CA).
2.9. Serum MCP-1
Serum concentrations of MCP-1 (n = 5 mice per group) were measured using a mouse-specific MCP-1 ELISA (Raybiotech, Norcross, GA), as described [51].
2.10. Histopathology
Mouse tissues were collected at necropsy (n = 3–8 mice per group) and placed in 10% neutral buffered formalin for 48 h, transferred to 70% alcohol, and subsequently processed into paraffin blocks for sectioning and hematoxylin and eosin staining. Tissue sections were scored for the presence and severity of a well-defined panel of age-related lesions by a veterinary pathologist to create a composite tissue lesion score for each animal that reflects healthspan, as previously described [52].
2.11. RNA isolation and qPCR
Tissues were harvested from mice (n = 4–10 mice per group) and snap frozen in liquid nitrogen. Total RNA was harvested from tissues and the expression of several markers of senescence was measured, as previously described in [51]. Total RNA was quantified using a Nanodrop spectrophotometer (Thermo Fisher, Waltham, MA) and 1 μg of total RNA was used to generate cDNA using the Transcriptor First Strand cDNA synthesis kit (Roche, Basel, Switzerland). Gene expression changes were carried out in 20 μL reactions using the Universal SYBR Green master mix with ROX (Roche) and a StepOne thermocycler (Thermo Fisher). Primers for the genes of interest are as follows: Cdkn1a (p21Cip1) Fwd 5’-GTCAGGCTGGTCTGCCTCCG-3′, Cdkn1a (p21Cip1) Rev. 5’-CGGTCCCGTGGACAGTGAGCAG-3′; Cdkn2a (p16Ink4a) Fwd 5′- CCCAACGCCCCGAACT-3′, Cdkn2a (p16Ink4a) Rev. 5′- GCAGAAGAGCTGCTACGTGAA-3′; Gapdh Fwd 5’-AAGGTCATCCCAGAGCTGAA-3′, Gapdh Rev. 5’-CTGCTTCACCACCTTCTTGA-3′; Il6 Fwd 5’-CTGGGAAATCGTGGAAT-3′, Il6 Rev. 5’-CCAGTTTGGTAGCATCCATC-3′; Mcp1 Fwd 5’-GCATCCACGTGTTGGCTCA-3′, Mcp1 Rev. 5’-CTCCAGCCTACTCATTGGGATCA-3′. Data were analyzed by ΔΔCt method and gene expression was normalized to Gapdh.
2.12. Isolation of peripheral blood CD3+ T lymphocytes
Blood was obtained from mice n = 4–10 mice per group) postmortem by cardiac puncture, immediately placed into 1/10th volume of 0.5 M EDTA, and gently mixed to prevent coagulation. Samples were centrifuged at 300 g for 10 min in a table top centrifuge. The supernatant was discarded and the cell pellet was resuspended in 1 mL ACK buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.4) and incubated at room temperature for 10 min to lyse red blood cells. The cells were spun down and ACK lysis repeated. The cells were spun down, washed with PBS, and resuspended in PBS containing 0.5% FBS and 2 mM EDTA. Fifty μL of CD3-Biotin conjugate (Miltenyi Biotech, San Diego, CA) were added to the cell suspension and incubated for 30 min on ice. The cells were centrifuged at 100 g for 10 min and washed twice in resuspension buffer. The cell pellet was resuspended in 500 μL of resuspension buffer and 100 μL of anti-biotin microbeads were added followed by a 15 min incubation on ice. The cells were washed twice and then resuspended in 500 μL of resuspension buffer and applied to a MACS column attached to a magnet. The cells were washed with 3 column volumes of resuspension buffer before elution. The cells were centrifuged and RNA isolation conducted using an RNeasy kit (Qiagen, Germantown, MD) according to the manufacturer's specifications. qPCR analysis of senescence markers was performed as indicated above.
2.13. Senescence-associated β-galactosidase (SA-β-gal) staining of tissue
Fresh fat tissues (n = 6–7 mice per group) were fixed and stained to detect senescence-associated β-galactosidase activity, as described [53].
2.14. Mass cytometry/CyTOF in adipose tissue
This high dimensional single-cell proteomics technique combines time-of-flight mass spectrometry with metal-labelling technology to detect up to 40 protein targets per cell [54,55]. A panel of antibodies based on cell surface markers and transcription factors (see Supplemental Table 1) was designed for CyTOF analysis of adipose tissues. Each antibody was tagged with a rare metal isotope and its function verified by mass cytometry according to the factory manual (Multi Metal labeling Kits, Fluidigm, CA). A CyTOF-2 mass cytometer (Fluidigm, South San Francisco, CA) was used for data acquisition. Acquired data were normalized based on normalization beads (Ce140, Eu151, Eu153, Ho165, and Lu175). One gram of subcutaneous fat tissue (n = 6–9 mice per group) was dissociated into a single-cell suspension using an adipose tissue dissociation kit (Adult Adipose Tissue Dissociation Kit, Miltenyi Biotec Inc.CA). Collected cells were incubated with metal-conjugated antibodies for cell surface markers and intracellular proteins. Fixation and permeabilization were conducted according to the manufacturer's instructions (Transcription Factor Staining Buffer Set, eBioscience, San Diego, CA). CyTOF data were analyzed by Cytobank (Santa Clara, CA).
2.15. Human adipose tissue explants
The protocol was approved by the Mayo Clinic Foundation Institutional Review Board for Human Research. Informed consent was obtained from all subjects (n = 3). Human greater omental adipose tissue was resected during surgery from 2 lean (BMI 25.5 and 26.2) and 1 obese (BMI 45.6) female subjects, ages ranging from 55 to 66 years. No subject was known to have a malignancy. The adipose tissue was cut into small pieces and washed with PBS 3 times. The adipose tissue was then cultured in medium containing 1 mM sodium pyruvate, 2 mM glutamine, MEM vitamins, MEM non-essential amino acids, and antibiotics with 20 μM of fisetin or DMSO. After 48 h, the adipose explants were washed 3 times with PBS and was then maintained in the same media without drugs for 24 h to collect conditioned medium (CM) for multiplex protein analysis. The adipose explants then were fixed and stained to detect senescence-associated β-galactosidase activity [85].
2.16. Multiplex protein analyses
Pro-inflammatory cytokine and chemokine protein levels in CM from the adipose tissue explants (n = 3) were measured using Luminex xMAP technology. The multiplexing analysis was performed using the Luminex™ 100 system (Luminex, Austin, TX) by Eve Technologies Corp. (Calgary, Alberta, Canada). Human multiplex kits were from Millipore (Billerica, MA). The secreted protein levels in CM were normalized to the tissue weights and plotted as a percent relative to the vehicle control.
3. Results
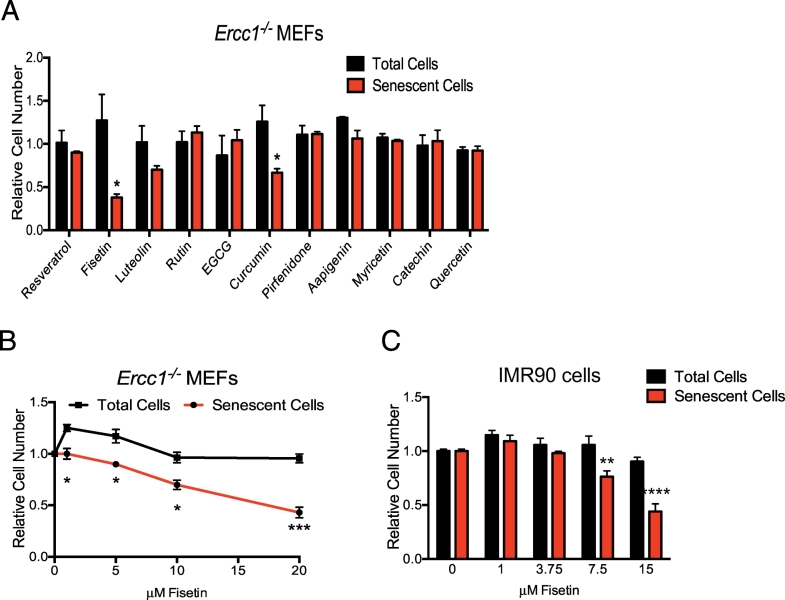
We previously demonstrated that the flavonoid quercetin, an antioxidant, which also targets PI3 kinase delta as well as certain BCL-2 family members, reduces senescence in primary human umbilical vein endothelial cells (HUVECs) and murine embryonic fibroblasts (MEFs), especially when used in combination with the tyrosine kinase inhibitor dasatinib [30]. To determine if other flavonoids might have more potent senotherapeutic activity than quercetin, a panel of flavonoids was screened for effects on senescence induced by oxidative stress [49]. Primary MEFs from Ercc1−/− mice were used. These cells undergo premature senescence if grown at atmospheric oxygen [56]. Ercc1−/− MEF cultures were established at 3% O2 then shifted to 20% O2 for three passages to induce senescence. To quantify senescent cells, SA-ß-gal activity was measured using the fluorescent substrate C12FDG [57] using an IN Cell Analyzer 6000 confocal imager. At a dose of 5 μM, fisetin was most effective in reducing the fraction of SA-ß-gal positive MEFs (Fig. 1A). Luteolin and curcumin also showed weak activity at a dose where quercetin was ineffective. In addition, fisetin reduced senescence in MEFs and IMR90 cells in a dose-dependent manner (Fig. 1B and C). These results are consistent with our previous finding that fisetin selectively reduces the viability of senescent HUVECs without affecting proliferating cells [32]. In HUVECs, fisetin induces apoptosis as measured by caspase3/7 activity, whereas in MEFs, fisetin suppressed markers of senescence without evidence of cell killing [32].
Identification of fisetin as a putative senolytic. (A) Passage 5 Ercc1−/− MEFs were treated with a panel of flavonoid compounds at a dose of 5 μM and the viability of senescent cells (SA-β-gal+ cells detected by C12FDG staining; red bars) and total cells (black bars) measured using an IN Cell Analyzer 6000. The number of viable cells is calculated relative to cells treated with vehicle only (DMSO). n = 3 independent experiments, one-way ANOVA. (B) Quantitation of the total number of viable Ercc1−/− MEFs and viable senescent Ercc1−/− MEFs after treating mixed cultures of proliferating and senescent cells with various doses of fisetin from two biological replicates conducted in triplicate. Two-tailed unpaired Student's t-test. (C) Early passage IMR90 cells were treated for 24 h with 20 μM etoposide. Two days after etoposide removal, ~70% of the cells were SA-β-gal+. Cells were treated for 48 h with different concentrations of fisetin (1–15 μM) and the percentage of SA-β-gal+ cells was determined using C12FDG, as described above. Graphed are the relative number of viable cells compared to cultures treated with vehicle only (DMSO). All samples were analyzed in duplicate with 3–5 fields per well and reported as the mean ± S.D. Two-tailed unpaired Student's t-test. Plotted is the mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
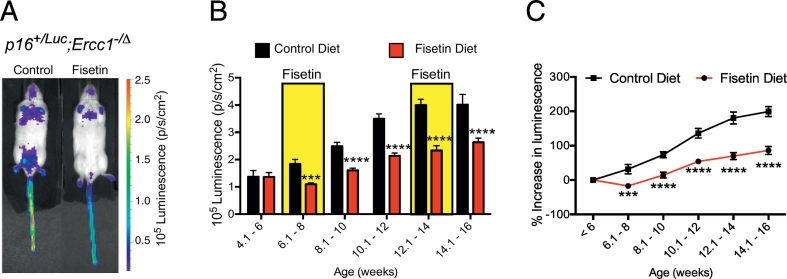
To test the senotherapeutic activity of fisetin in vivo, initially progeroid Ercc1−/∆ mice carrying a p16Ink4a-luciferase reporter transgene were used [47,50]. These mice show accelerated accumulation of senescent cells compared to WT mice, but the overall level of senescence never exceeds that of naturally aged mice [50]. Ercc1−/∆;p16Ink4a-luciferase mice were fed a standard Teklad 2020 chow diet with or without supplementation with 500 ppm (500 mg/kg) of fisetin, ad libitum (approximately 60 mg/kg fisetin per day). The mice were exposed to a fisetin diet intermittently from 6 to 8 then 12–14 wks of age. Whole body luciferase activity was measured before starting the fisetin diet then weekly thereafter. Animals in the two treatment groups had an equivalent luciferase signal prior to administration of the experimental diet. Dietary fisetin suppressed the luciferase signal of Ercc1−/∆;p16Ink4a-luciferase mice significantly (Fig. 2A-B). The luciferase signal was lower at every time point after initiation of the fisetin diet (Fig. 2B-C). Notably, the level of p16Ink4a expression remained significantly lower in the fisetin-treated mice throughout the 4 week period when the animals were not exposed to fisetin (8–12 wks of age, Fig. 2B). This is consistent with a mechanism of action where senescent cells are cleared (senolytic) or senescence is reversed (senomorphic) but not a mechanism in which fisetin must be chronically present to suppress senescence.
Intermittent treatment of progeroid mice with fisetin reduces senescent cell burden. (A) Representative image of age-matched, 12 week-old male p16+/Luc;Ercc1−/∆ mice fed a diet containing 500 ppm (500 mg/kg) fisetin, or drug-free control diet. (B) Luciferase signal was measured biweekly in p16+/Luc;Ercc1−/∆ mice fed either control chow or chow containing 500 ppm fisetin, n = 4–10 mice per group and time point. The fisetin was administrated intermittently for two weeks at a time (yellow bars). Otherwise the mice were fed a control diet. (C) The same data as seen in (B), but plotted as the percent change in luciferase signal as the animals aged. Values represented as the mean ± SEM. Two-tailed unpaired Student's t-test. ***p < .001, ****p < .0001.
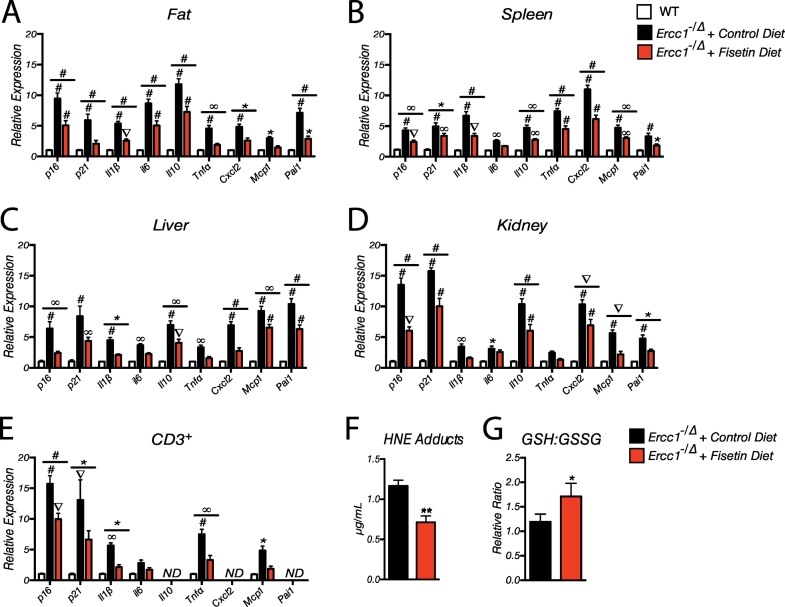
To validate the imaging data, Ercc1−/∆ mice were treated with the 500 ppm fisetin diet for 10 wks beginning at 10 wks of age, then tissues collected for measurement of multiple markers of senescence including p16Ink4a and p21Cip1 and SASP factors. Expression of p21Cip1 was included since not all senescent cells are p16Ink4a positive: some are p21Cip1 positive, but p16Ink4a negative. As shown in Fig. 3A-D, p16Ink4a and p21Cip1 mRNA, as well as SASP markers, were significantly elevated in the fat, spleen, liver, and kidney of Ercc1−/∆ mice compared to age-matched WT mice. Fisetin reduced expression of senescence and SASP markers significantly in all tissues. Similarly, there was a reduction in the expression of p16Ink4a, p21Cip1 and the SASP factors in peripheral blood CD3+ T cells (Fig. 3E), a cell type that demonstrates a robust increase in p16INK4a expression as humans age [58]. In addition, fisetin reduced oxidative stress in the liver as determined by measuring the lipid peroxidation product 4-hydroxynonenal (HNE) adducts and an increase in the ratio of reduced to oxidized glutathione (Fig. 3F-G), consistent with data indicating fisetin has antioxidant activity as well as increasing intracellular glutathione [45].
Chronic fisetin treatment reduces senescence in progeroid mice. (A-D) Tissues from 20-week-old male and female Ercc1−/∆ mice chronically exposed to fisetin through their diet or fed a control diet were analyzed for the expression of senescence (p16Ink4a and p21) and senescence-associated secretory phenotype (SASP) (Il1β, Il6, Il10, Tnfα, Cxcl2, Mcp1, and Pai1) markers using qRT-PCR. Age-matched WT mouse tissues were used to normalize expression. n = 4–10 mice per group. Graphed is the mean ± S.E.M. One-way ANOVA with Tukey's multiple comparison test. (E) Quantitation of senescence and SASP markers in CD3+ peripheral T cells isolated from the same mice. mRNA levels were measured by qRT-PCR. (F) 4-hydroxynonenal (HNE) adducts were measured in liver (n = 4–5 mice per group) by ELISA as an index of oxidative stress. (G) Reduced (GSH) and oxidized glutathione (GSSG) were quantified in liver (n = 4–5 mice per group) as an index of antioxidant buffering capacity. Differences were assessed by two-tailed unpaired Student's t-test. Values represented as the mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .0001.
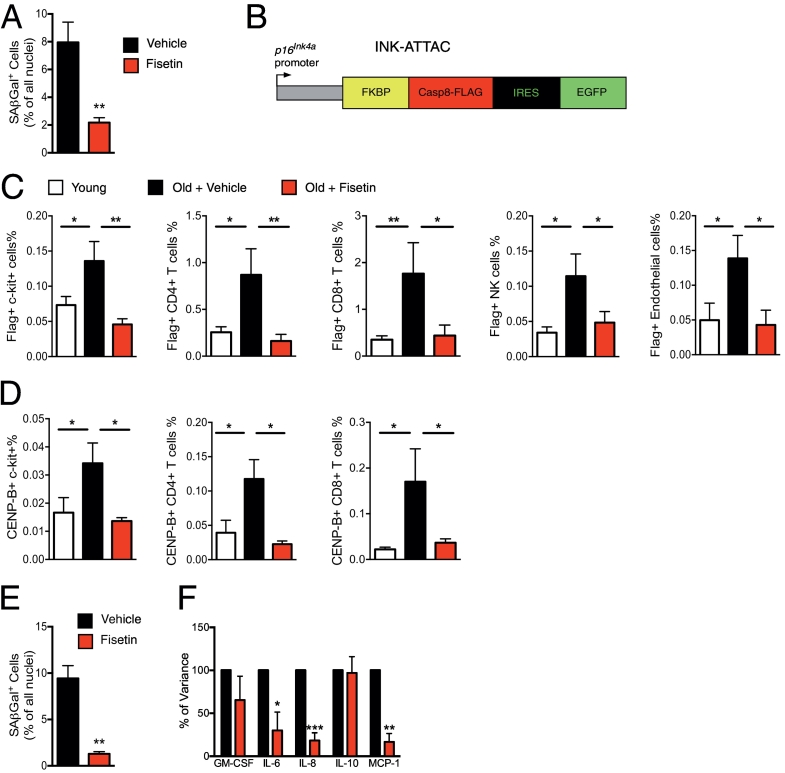
To confirm further the data obtained in progeroid mice, we employed naturally aged C57BL/6 mice and different methods of detecting senescence in tissue. 22–24-month-old mice were treated with 100 mg/kg fisetin for 5 consecutive days by oral gavage, or vehicle only. Mice were sacrificed 3 days after the last dose and the number of SA-ß-gal+ cells present in inguinal fat was determined by staining tissue sections to measure SA-β-gal activity. Fat tissue was chosen for the analysis since there is a clear upregulation of senescence markers including SASP in our mouse models, the tissue has a significant increase in the fraction of senescent cells including senescent immune cells, such as T and endothelial cells and macrophages, and injection of senescent pre-adipocytes is sufficient to induce frailty in young mice [26,50,59,60]. Short-term treatment with fisetin significantly reduced the fraction of senescent cells in white adipose tissue (WAT). To determine which cells become senescent in WAT and which cell types are cleared by fisetin, CyTOF analysis was performed on subcutaneous adipose tissue from aged INK-ATTAC mice expressing a Flag-tagged FKBP-Casp8 protein from the p16Ink4a promoter (Fig. 4B). The Flag tag enabled identification of senescent (p16Ink4a-expressing) cells using an anti-Flag antibody. CyTOF analysis revealed a significantly elevated fraction of senescent cells in fat from old mice compared to young and identified these cells as mesenchymal stem/progenitor cells, T lymphocytes, natural killer cells, and endothelial cells (Fig. 4C). The short-course treatment with fisetin resulted in a significant reduction in the fraction of senescent cells in each of these populations (Fig. 4C). Fisetin reduced the fraction of p16Ink4a-expressing, c-Kit+ stem/progenitor cells, CD4+ and CD8+ T cells, NK-1.1+ NK cells, and CD146+CD31+ endothelial cells (Fig. 4C). These data are also shown in Spanning-tree Progression Analysis of Density-normalized Events (SPADE) analysis (Supplemental Fig. 1). To confirm senescence in these cell populations, CENP-B protein was measured by CyTOF (Fig. 4D). CENP-B binds centromeric satellite DNA [61], which becomes distended in senescent cells. The fraction of CENP-B+ cells in WAT was significantly increased in old mice compared to young and suppressed by treating the mice with a short-course of fisetin, in the same manner as p16Ink4a-expressing/FLAG+ cells. In contrast, p21Cip1 (another cell-cycle regulator that is often up-regulated in senescent cells) expression was not significantly elevated in these cell populations (Supplemental Fig. 2). While FLAG+ dendritic cells and macrophages were increased in WAT from old mice consistent with a previous report [62], fisetin treatment had no substantial effect on the fraction of macrophages or dendritic cells with high p16Ink4a, CENP-B, or p21Cip1 (Supplemental Fig. 3). Taken together, these data demonstrate that a short-course of fisetin reduces the number of p16Ink4a-expressing cells in subcutaneous WAT including mesenchymal stem/progenitor, immune, and endothelial cells. This is the first time a senotherapeutic has been demonstrated to differentially affect senescent cells of different lineages in vivo.
Acute fisetin treatment reduces senescent cell burden in aged wild-type mice and human explants. (A) 22–24-month-old WT C57BL/6 mice were given fisetin (100 mg/kg) or vehicle for 5 days by oral gavage. 72 h after the final dose, the mice were sacrificed and the SA-β-gal+ cells were quantified in inguinal fat (n = 6–7 mice per group). Two-tailed unpaired Student's t-test, **p < .01. (B) Schematic diagram of the INK-ATTAC transgene [25]. Expression of FLAG-tagged FKBP-Caspase-8 protein is driven by the p16Ink4a promoter enabling detection of p16-expressing cells in tissues using immunodetection of FLAG. (C) Aged INK-ATTAC male mice (22–24 months) were acutely treated with fisetin as described above and CyTOF analysis used to quantify p16Ink4a/FLAG+ cell populations in subcutaneous fat tissue (c-kit+ mesenchymal stem cells, CD4+ and CD8+ T cells, NK-1.1+ NK cells, and CD146+CD31+ for endothelial cells). Subcutaneous fat tissue from 6 month-old male mice was used as a control. (D) Quantification of another marker of cellular senescence in the same cell populations (CENP-B+ cells). The data are plotted as the mean ± SEM based on n = 9 mice per group. One-way ANOVA with Tukey's multiple comparison test. (E) Human adipose tissue explants (n = 3) were treated with 20 μM fisetin for 48 h, then washed and placed in fresh media for 24 h in order to condition the media. The adipose tissue explants were then stained to measure the percent of SA-ß-gal+ cells. (F) Cytokine and chemokine levels were measured in the conditioned media from the adipose tissue explants using multiplex protein analyses and normalized to adipose tissue weight (n = 3 biological replicates). The results are plotted as the percent expression of various cytokines relative to samples from the same individual treated with vehicle only. Two-tailed paired Student's t-test. Values represented as the mean ± SEM. *p < .05, **p < .01, ***p < .001.
To determine if fisetin also reduces senescence in human adipose tissue, greater omental adipose explants resected during surgery were treated with fisetin ex vivo. The tissue explants were treated for 48 h with 20 μM fisetin, washed, and cultured for an additional 24 h before measuring SASP factors by multiplex protein analysis [26]. Fisetin treatment caused a significant reduction in the percent of SA-ß-gal positive cells (Fig. 4E) as well as in expression of the SASP factors IL-6, IL-8, and MCP-1 in human WAT (Fig. 4F). These data support the translational potential of fisetin to reduce senescent cell burden and associated inflammation.
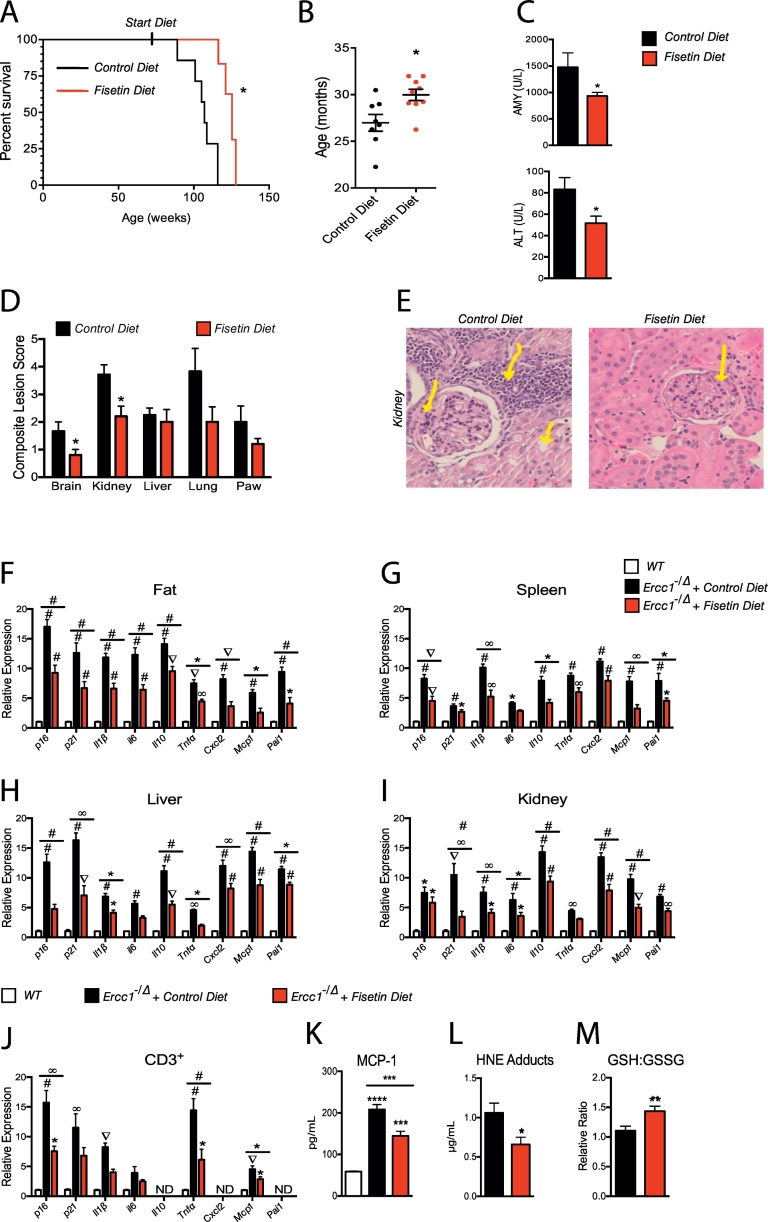
To determine if fisetin-mediated clearance of senescent cells impacts the health or lifespan of mice, WT f1 C57BL/6:FVB mice were fed a diet containing 500 ppm fisetin beginning at 85 wks of age, roughly equivalent to age 75 years in humans. This resulted in an extension of median as well as maximal lifespan (Fig. 5A-B). Amylase and alanine aminotransferase (ALT) were significantly lower in serum of aged WT mice fed the diet supplemented with fisetin, consistent with improved pancreatic and liver homeostasis (Fig. 5C). Brain, kidney, liver, lung, and forepaw tissue sections were stained with hematoxylin and eosin and evaluated by a veterinary pathologist. Using the Geropathology Grading Platform to score age-related lesions [63], several tissues had reduced age-related pathology in the fisetin diet group compared to the control diet (Fig. 5D). An example of this is illustrated in a representative image from renal sections in Fig. 5E. Similar to the progeroid mice, fisetin reduced the expression of senescence and SASP markers in multiple tissues of aged WT mice exposed to oral fisetin (Fig. 5F-I). Furthermore, there was a reduction in senescence and SASP factor expression in peripheral CD3+ T cells (Fig. 5J). There was also a reduction in levels of circulating MCP-1 (Fig. 5K), a SASP factor [51]. Finally, fisetin reduced oxidative stress in the liver of old WT mice (Fig. 5L-M).
Late-life intervention with fisetin in aged wild-type mice extends health span and lifespan. (A) At 85-weeks of age (>20 mth), male and female mice were administered a diet containing 500 ppm (500 mg/kg) fisetin or fed a control diet with no drug. Lifespan was measured. n = 8–9 mice per group. Log rank (Mantel-Cox) test. (B) Median lifespan of the same cohort of mice. Each dot represents an individual animal. Black bars indicate the mean ± S.E.M. Two-tailed unpaired Student's t-test. (C) Clinical chemistry on blood from the above mice to measure markers of liver (alanine aminotransferase/ALT) and pancreatic (amylase/AMY) dysfunction. n = 3–6 mice per group. Two-tailed unpaired Student's t-test. (D) Composite lesion scores for aged-related pathologies in multiple tissues determined by histopathologic analysis according to the criteria of the Geropathology Grading Platform [63]. n = 3–8 mice per group. Two-tailed unpaired Student's t-test. (E) Representative images of the kidney of a mouse fed control chow or fisetin chow. In the control mouse, arrows (from left to right) indicate increased cellularity at a segment of the glomerular capsule border, moderate levels of lymphoid aggregates, and tubular cell vacuolization. In the fisetin-treated mouse, the arrow indicates only mild segmental cellularity at the glomerular capsule border and a few scattered lymphoid cells near the glomerulus (200× magnification). (F-I)—Tissues from >120-week-old mice (~30 mth) fed control or fisetin chow were analyzed for the presence of senescence (p16Ink4a and p21) and senescence-associated secretory phenotype (SASP) (Il1β, Il6, Il10, Tnfα, Cxcl2, Mcp1, and Pai1) markers by qRT-PCR. Results are expressed as a function of values in 16–18-week-old “Young” WT mice. n = 4–10 mice per group. One-way ANOVA with Tukey's multiple comparison test. (J) Senescence and SASP marker expression were measured in CD3+ peripheral T cells by qRT-PCR. Results are expressed as a function of values in 16–18-week-old “Young” WT mice. n = 4–6 mice per group. One-way ANOVA with Tukey's multiple comparison test. (K) Circulating levels of the SASP factor chemokine MCP-1 were measured by ELISA. n = 5 mice per group. One-way ANOVA with Tukey's multiple comparison test. (L) 4-hydroxynonenal (HNE) adducts a marker of lipid peroxidation and oxidative stress measured by ELISA in liver. n = 5–6 mice per group. Two-tailed unpaired Student's t-test. (M) The ratio of reduced (GSH) to oxidized (GSSG) glutathione was measured as an index oxidative stress. n = 6–7 mice per group. Values represented as the mean ± SEM. Two-tailed unpaired Student's t-test. *p < .05, **p < .01, ***p < .001, ****p < .0001.
4. Discussion
Aging is a complex process involving numerous pathways and both genetic and environmental components [[64], [65], [66], [67], [68], [69]]. The biological processes that drive the aging process contribute to the etiology of most chronic diseases including: 1) chronic, “sterile” inflammation; 2) macromolecular changes in proteins, carbohydrates, lipids, mitochondria, and DNA; 3) stem cell and progenitor dysfunction; and 4) increased cellular senescence [5,70]. These processes are linked in that interventions that target one appear to attenuate others. For example, senescent cells accumulate with age and at sites of pathogenesis in chronic diseases [5,70]. Reducing senescent cell burden can lead to reduced inflammation, decreased macromolecular dysfunction, and enhanced function of stem/progenitor cells [1,3,19]. Adult stem cells also become dysfunctional with age, displaying evidence of senescence [71]. We previously demonstrated that the combination of dasatinib and quercetin function as a senolytic in vivo, alleviating many age-related diseases [26,30]. Because of the senotherapeutic activity of quercetin, we examined other natural flavonoids for effects on senescent cells in hopes of improving therapeutic efficacy.
Here, we demonstrate that when tested against a panel of other flavonoids, fisetin had the most potent senotherapeutic effects in several cell types in vitro and showed strong anti-geronic effects in vivo. We demonstrated that acute (oral) or chronic (dietary) treatment of progeroid and WT mice with fisetin reduces markers of senescence and senescence-associated secretory phenotype in multiple tissues (Fig. 3A-D, A-D,4A,4A, A,5F-I).5F-I). These data were collected in two labs using progeroid and WT mice in two distinct genetic backgrounds. Specifically, p16Ink4a-expressing or CENP-B+ mesenchymal stem/progenitor, T lymphocytes, natural killer and endothelial cells were removed from subcutaneous fat of old mice, but not activated senescent-like macrophages or dendritic cells. The effect of fisetin was greater on p16Ink4a-expressing cells than on p21CIP1-expressing cells, at least in subcutaneous fat (Fig. 4). Our findings reveal that fisetin targets multiple, but not all types of senescent cells in vivo. Furthermore, by reducing the percent of senescent cells, fisetin reduces expression of senescence markers in multiple organs as measured by qPCR. This results in improved tissue homeostasis and reduction in multiple age-related pathologies, consistent with effects on a fundamental aging process.
The fact that fisetin reduced the fraction of senescent T and NK cells could help amplify the beneficial effects of fisetin, since healthy immune cells are important for clearing senescent cells [72,73]. Similarly, fisetin reduces markers of inflammation and oxidative stress (Figs. 3F-G and and5L-M),5L-M), consistent with prior literature [45]. This too could contribute to the reduction in senescence markers. The decrease in these markers was observed in tissues harvested several days after completion of fisetin administration. Since the rapid and terminal half-lives of fisetin are 0.09 and 3.1 h respectively [74], these improvements did not depend on continued presence of circulating fisetin. This is more consistent with fisetin causing removal of senescent cells, which take days to weeks to form after an insult (at least in culture), than with effects exerted by continued occupancy of a receptor or effects on an enzyme. However, it is important to note that given the multiple reported activities of flavonoids like fisetin, it is also possible that the extension of healthspan is due to mechanisms in addition to the reduction in senescence, such as altering the gut microbiome [75].
Fisetin extends the replicative lifespan of S. cerevisiae by 55% [76] and the lifespan of D. melanogaster by 23% [77]. Here, we show for the first time a similar effect in vertebrate animals. Chronic exposure to fisetin improves healthspan and extends the median and maximum lifespan of mice. Importantly, in our study fisetin supplementation was initiated in mice >20 months old. This increases the translational potential of our study since senotherapeutic interventions are most feasibly administered in elderly humans after the onset of age-related diseases, rather than in younger asymptomatic subjects, in whom any side-effects would be unacceptable.
Fisetin is a member of the flavonoid family, a family of naturally occurring polyphenolic compounds. Fisetin, a high Trolox-equivalent antioxidant, is present in low concentrations in many fruits and vegetables such as apples, persimmon, grapes, onions, and cucumbers and at higher concentrations in strawberries [43,44]. The average dietary intake of naturally occurring fisetin in Japan is approximately 0.4 mg/day [78,79], apparently without any adverse effects. Fisetin has anti-cancer activity and appears to block the PI3K/AKT/mTOR pathway [80]. We previously found that transiently disrupting the PI3K/AKT pathway by RNA interference leads to death of senescent cells [30], as with other SCAPs that defend senescent cells from their own pro-apoptotic SASP [28,29]. Fisetin, like some other flavonoids, is a topoisomerase inhibitor, which may also contribute to its anti-cancer activity [81]. It increases the catalytic activity of hSIRT1 at least in vitro [76]. Also in vitro, fisetin inhibits the activity of several pro-inflammatory cytokines, including TNFα, IL-6, and the transcription factor NF-κB [82]. Fisetin has direct activity as a reducing agent, chemically reacting with and neutralizing reactive oxygen species [83,84]. Fisetin scavenges free radicals as a result of its electron donating capacity, which is due to the presence of two hydroxyl groups on one ring and a third hydroxyl group on another ring. Fisetin also upregulates synthesis of glutathione, an endogenous antioxidant [43,82]. Other biological activities include anti-hyperlipidemic [[84], [85], [86]], anti-inflammatory [85], and neurotrophic [87] effects, some of which could be mediated through a reduction in the senescent cell burden, particularly since when administered intermittently, fisetin alleviated dysfunction despite its short elimination half-life, more consistent with elimination of senescent cells than action on a receptor or enzyme requiring continuous drug presence.
The chemical structure of fisetin is almost the same as quercetin except for a hydroxyl group in position 5. Thus, it is highly likely that these two closely related compounds exert many similar effects. Interestingly, preliminary medicinal chemistry on fisetin has identified analogues with enhanced senotherapeutic activity, suggesting that even more effective flavonoids can be developed for extending healthspan with minor alterations in the structure of fisetin.
Given that fisetin is a natural product found in common foods and available as an oral dietary supplement and has no reported adverse side effects [45], our pre-clinical data suggest that fisetin should be imminently translatable and could have a significant benefit to the health of elderly patients. Based on these mouse studies, clinical trials to evaluate the short-term benefits of intermittent fisetin treatment on certain aspects of aging such as frailty are currently underway.
Author contributions
M.J.Y. and J.I.K. performed the analyses of senescence and SASP markers in the tissues of the treated mice; Y.Z. performed the CyTOF analyses with oversight and analysis by E.A.A.; S.J.M., K.M., E.A.W., C.M. and L.A. performed the mouse treatment studies at The Scripps Research Institute; T.P. and C.L.I. performed the mouse studies at Mayo Clinic; H.F., D.G; and Y.Y.L. performed the testing of flavonoids in cell culture; M.X. performed the analysis in human adipose tissue explants; W.L.L performed histopathology analyses; C.E.B. provided the p16INK4a-Luc+/− mice and assisted with analyses; T.T., J.L.K., E.A.A., P.D.R., and L.J.N. oversaw all experimental design, data analysis, and manuscript preparation.
Competing financial interests
J.L.K, T.T., Y.Z., M.X., and T.P. have a financial interest related to this research. Patents on senolytic drugs (PCT/US2016/041646) are held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. None of the other authors has a relevant conflict of financial interest.
Footnotes
Appendix ASupplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.09.015.