- Journal List
- HHS Author Manuscripts
- PMC5826793

Ketogenic diet-induced extension of longevity in epileptic Kcna1-null mice is influenced by gender and age at treatment onset
Kyoung-chul Chun
aDepartments of Neurobiology and Neurology, Barrow Neurological Institute, St. Joseph’s Hospital & Medical Center, Phoenix, Arizona, USA
bDepartments of Obstetrics and Gynecology, College of Medicine, Inje University, Ilsan-Paik Hospital, Gyeonggi, Korea
Shun-Chieh Ma
aDepartments of Neurobiology and Neurology, Barrow Neurological Institute, St. Joseph’s Hospital & Medical Center, Phoenix, Arizona, USA
Hyoungil Oh
aDepartments of Neurobiology and Neurology, Barrow Neurological Institute, St. Joseph’s Hospital & Medical Center, Phoenix, Arizona, USA
Jong M. Rho
cDepartments of Pediatrics, Clinical Neurosciences, Physiology & Pharmacology, Alberta Children’s Hospital Research Institute, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada
Do Young Kim
aDepartments of Neurobiology and Neurology, Barrow Neurological Institute, St. Joseph’s Hospital & Medical Center, Phoenix, Arizona, USA
Abstract
Sudden unexpected death in epilepsy (SUDEP) is a leading cause of premature mortality in patients with epilepsy, and has been linked to multiple risk factors, including gender and early age at seizure onset. Despite the lack of a targeted therapy for SUDEP, it has recently been shown that a high-fat, low carbohydrate ketogenic diet (KD) enhances longevity in the epileptic Kcna1-null (KO) mouse, a validated model of SUDEP. Here, we asked whether the KD-driven prolongation of lifespan in KO mice is dependent on sex and/or age at treatment onset. We found that as KO mice aged, their daily seizure frequency steadily increased, but had early demise by postnatal day (PD) 46.9 ± 0.8. In KO mice started on the KD at PD30, longevity was extended to a mean of PD69.8 ± 1.7, accompanied with improved seizure control. Interestingly, while seizure control on the KD was similar between male and female mice, KD-fed female KO mice survived longer than their male counterparts. Further, epileptic mice initiated on the KD at PD25 had longer lifespans compared to those placed on the KD starting at PD35. Collectively, these data further support the notion that the KD can retard disease progression and sudden death in KO mice, but that this beneficial action is influenced by gender and age at the start of treatment.
INTRODUCTION
Sudden unexpected death in epilepsy (SUDEP) is a clinically significant problem – affecting as many as 1 per 1000 patients annually (Maguire et al., 2016; Surges et al., 2009). The clinical hallmark feature of SUDEP appears to be cardiorespiratory compromise associated with a terminal generalized tonic-clonic (GTC) seizure (Surges et al., 2009). To date, several risk factors for SUDEP have been identified, including GTC frequency, gender, early age at seizure onset and polytherapy with anti-seizure drugs (ASDs) (Maguire et al., 2016; Nilsson et al., 1999). Given the profound impact of SUDEP in patients with medically intractable epilepsy, the development of therapeutic strategies to prevent this catastrophic outcome is of paramount importance.
Among the few clinically relevant animal models of SUDEP is the Kcna1-null mutant (KO) mouse lacking the delayed rectifier potassium channel α subunit Kv1.1 (Glasscock et al., 2010; Simeone et al., 2016). These KO mice manifest severe spontaneous recurrent seizures (SRS) beginning early in postnatal development and experience premature sudden death. Recently, the high-fat ketogenic diet (KD), a clinically proven therapy for patients with treatment-resistant epilepsy, was shown to extend lifespan in KO mice (Simeone et al., 2016). To delineate specific experimental variables impacting this protective effect, we asked whether gender and/or age at initiation of dietary treatment might affect longevity.
METHODS
Animals
Spontaneously epileptic KO mice were generated using heterozygous breeding pairs in the animal facility at the Barrow Neurological Institute (BNI). Pups were genotyped by PCR analysis of tail genomic DNA. After weaning at postnatal day (PD) 18–21, mice were fed either a ketogenic (KD) Bio-Serv F3666 diet (Flemington, New Jersey, USA; 6.3:1 ratio of fats to carbohydrate plus protein by gross weight) or normal standard diet [SD, % of calories: 17 (fat), 18 (protein), 63 (Carbohydrate)]. It should be noted that the macronutrient composition of the SD compared to Western diet [30–35% fat] is somewhat different. All animal handling protocol was approved by the Institutional Animal Care and Use Committee at the BNI and St. Joseph’s Hospital & Medical Center.
Seizure monitoring
Both normal light and infrared video cameras were used to record behavioral activity in each mouse continuously for 24 hours/day. Behavioral seizures were scored using a modified Racine scale: Stage 1, immobility; Stage 2, head bobbing; Stage 3, forelimb/hindlimb clonus, a lordotic posture, and tail extension; Stage 4, rearing and falling; Stage 5, tonic-clonic seizures with loss of the righting reflex. In this study, we defined Stages 3–5 as epileptic behaviors.
β-hydroxybutyrate (BHB) measurement
BHB levels were measured in blood samples from tail clippings using a Precision Xtra™ ketone meter, which was calibrated with standard solutions including known concentrations of BHB.
Statistical analysis
Numerical data were expressed as the mean ± the standard error of the mean (SEM). Either the t-test or one-way ANOVA with Tukey test were used to assess differences amongst experimental groups. Kaplan-Meier analysis and a Wilcoxon test were used to plot survival curves.
RESULTS
KD administration affords seizure suppression and lifespan extension in KO mice
A growing body of evidence has supported the anti-seizure effects of the KD in epileptic brain (Kim et al., 2015). Given that deletion of the Kv1.1 potassium channel protein results in spontaneous recurrent seizures (SRS) and death early in life (Kim et al., 2015; Simeone et al., 2016), we asked whether the KD might also affect the early mortality. In our hands, SD-fed KO mice experienced sudden unexpected death at PD 46.9 ± 0.8 (n=112) (Fig. 1A). As mice grew older, KO mice exhibited an increase in SRS, with a daily seizure frequency of 12 ± 2.8 at PD40-45 (Fig. 1C). When KO mice were treated with the KD starting at PD30, the mean daily seizure frequency decreased to 4.1 ± 0.9 between PD40-45, and the average age at the time of sudden death was prolonged to PD69.8 ± 1.7 (n=36) (Fig. 1B &C). Nonetheless, despite an overall lower seizure frequency in KD-fed KO mice during aging, the KD failed to block a terminal GTC-driven sudden death.
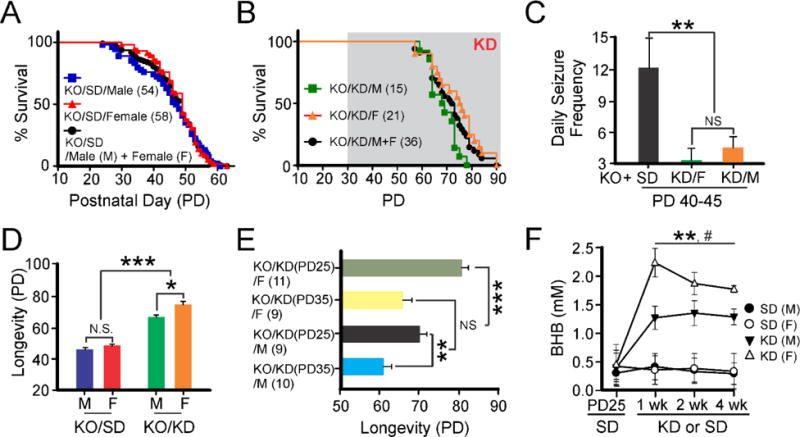
Ketogenic diet (KD)-induced longevity extension in Kcna1-null (KO) mice. (A) There are no gender differences in SUDEP risk in SD-fed KO mice. (B) Following KD treatment starting at PD30, KD-fed KO mice survived longer than SD-fed KO mice, and this effect was more pronounced in KD-fed female KO mice compared to male counterparts. (C) Summary of daily seizure frequencies in KO mice with or without the KD treatment between PD40-45. (D) Summary bar graph of the different experimental groups showing sex-dependent lifespan effects. (E) Summary data indicating effects on lifespan of KO mice as a function of age at initiation of the KD (PD25 vs. PD35) and gender. (F) KD-fed KO mice exhibited significant increases in blood BHB levels compared to SD-fed mice (N=7 per group; ** p <0.01). The pound sign reflects significant differences between KD-fed female mice and KD-fed male mice (p < 0.05). Each symbol indicates the mean ± standard error of the mean. One-way analysis of variance followed by Tukey test or t-test: *, # p < 0.05, ** p <0.01, *** p <0.001 and NS, not significant.
Gender and age at dietary treatment onset affect longevity of KO mice
In SD-fed KO animals, there was no difference in lifespan between male mice (n=54; PD45.7 ± 13) and female mice (n=58; PD48.1 ± 0.9) (Fig. 1A&D). Although not statistically significant, there was a trend toward decreased seizure frequency in KD-fed female mice (Fig. 1C). Thus, KD-fed female KO mice lived longer than males (PD73.1 ± 2.5 [n=21] and PD65.1 ± 1.4 [n=15], respectively; p < 0.05) (Fig. 1D). Next, we assessed whether the age of KD initiation affected longevity of KO mice. KO mice started on the KD at PD25 (n=20) had longer lifespans than those started at PD35 (n=19) (Fig. 1E). Thus, changes in longevity were dependent on both gender and age at KD initiation (Fig. 1E). Interestingly, while all KD treatment groups showed elevations in blood BHB levels, female KO mice (n=11) had significantly higher BHB concentrations compared to male counterparts (n=9) (p <0.05; Fig. 1F).
DISCUSSION
The principal finding of this study is that KD treatment significantly prolongs the lifespan of epileptic KO mice, and with a concomitant reduction in seizure activity and consistent ketonemia. Despite lack of direct clinical evidence about the efficacy of the KD in SUDEP in humans, these results are consistent with a recent report demonstrating the beneficial effects of the KD in the same animal model of SUDEP (Simeone et al, 2016). The current study extends this earlier finding by demonstrating that female KO mice live longer in response to the KD, and exhibit higher levels of ketosis, relative to comparably aged male epileptic animals. Further, the lifespan extension induced by the KD is more pronounced when dietary treatment is started at an earlier age. Collectively, our data support the notion that metabolism-based treatments such as the KD might represent a readily available avenue to help prevent sudden death in epilepsy and highlight two factors that could potentially influence outcome.
Although earlier retrospective clinical data addressing the interaction between gender and sudden unexpected death risk indicated that male patients were more susceptible to SUDEP (Nilsson et al., 1999), there were no sex differences in our KO mice fed a SD. However, lifespan in KO mice was extended to a greater degree when the KD was administered earlier in life, and this effect was more pronounced in female animals. The underlying basis for this observation remains unclear, but it is possible that this is due to a better response in terms of seizure improvement to the KD. Consistent with this notion is a recent retrospective clinical study that reported improved seizure control in female, compared to male, patients while on the KD (Agarwal et al, 2017). This potential sex difference requires further investigation since previous clinical observations failed to reveal gender as a critical factor in seizure control with the KD (Schoeler et al., 2013).
One interesting observation in the present study is that differences in longevity of KD-fed KO mice based on gender correlated with BHB levels. It is intriguing to speculate that higher levels of BHB may afford better seizure control (Kim et al., 2015), although the relationship between ketone levels and the clinical efficacy of the KD remains unclear. Furthermore, there has been growing interest in the effects of BHB on longevity in general (Veech et al., 2017). Lifespan prolongation may involve epigenetic mechanisms, such as modulation of histone deacetylases, mammalian target of rapamycin (mTOR), and/or sirtuins (McDaniel et al., 2011; Shimazu et al., 2013; Moore et al., 2014; Zhao et al., 2017).
One-third of individuals with epilepsy continue to have uncontrolled seizures despite taking multiple ASDs. In this population of patients, both early age of seizure onset and polytherapy with AEDs have been identified as potential risk factors for SUDEP (Maguire et al., 2016; Nilsson et al., 1999). In addition, there are genetic risk factors for premature sudden death in epilepsy. For example, mutations in the human KCNA1 gene encoding the Kv1.1 channel confers increased risk (Klassen et al., 2014), and loss of one allele may pose an intermediate risk, as evidenced by heterozygous Kv1.1 mice having a higher predisposition for seizures (Rho et al., 1999). However, following KD treatment beginning at both PD25 and PD35, we found that Kcna1-null mice showed enhanced longevity, but this beneficial effect was more striking in group started at PD25. Since PD25-35 in mice may be equivalent to human age range between 6 months and less than 10 years (Dutta et al., 2016), this age-dependent outcome is of potential clinical importance. Further clinical studies addressing mortality rates and seizure control in patients with SUDEP with and without KD treatment are necessary to validate our experimental findings.
Although the pathophysiological mechanisms underlying SUDEP remain unclear, one possibility is that seizure activity could spread into the medullary respiratory and autonomic centers, precipitating cardiac bradyarrhythmias due to increased parasympathetic tone (Glasscock et al., 2010; Moore et al., 2014). It is unknown whether KD administration influences SUDEP-related cardiac dysrhythmias, but long-term treatment with this diet in children with medically intractable epilepsy does not appear to induce detrimental effects on ventricular function (Ozdemir et al., 2016). Further, there is growing evidence that the KD imparts potential cardioprotective effects against ischemic insults as well as longevity extension, possibly through regulation of mitochondrial redox state and phosphorylation (Al-Zaid et al., 2007; Krebs et al., 2011).
In summary, our data further support the notion that the KD can prolong survival of epileptic Kcna1-null mice (that have previously been shown to expire from SUDEP), and that this effect is dependent on sex and age at initiation of therapy. The profound alterations in cellular metabolism and mitochondrial bioenergetics elicited by the KD are likely to be influenced by other factors as well, and future studies are necessary to clarify the specific mechanisms underlying the disease-modifying effects of the KD. Greater fundamental knowledge about KD-driven longevity extension will then critically inform novel therapeutic approaches to prevent SUDEP.
Highlights
- The ketogenic diet extends longevity in the Kcna1-null mouse, a model of sudden unexpected death in epilepsy
- Ketogenic diet-fed female knockout mice survive longer than their male counterparts
- Longevity extension is more pronounced when the ketogenic diet is introduced earlier after seizure onset
Acknowledgments
The authors would like to thank Daniel Rho and Derek O’Neill for technical assistance. This work was supported by the Barrow Neurological Foundation (DYK), the National Institutes of Health RO1 NS 070261 (DYK/JMR), and the Canadian Institutes of Health Research (JMR/DYK). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
None of the authors have any conflicts of interest to disclose.
References
- Agarwal N, Arkilo D, Farooq O, Gillogly C, Kavak KS, Weinstock A. Ketogenic diet: Predictors of seizure control. SAGE Open Med. 2017;5 2050312117712887. [PMC free article] [PubMed] [Google Scholar]
- Al-Zaid NS, Dashti HM, Mathew TC, Juggi JS. Low carbohydrate ketogenic diet enhances cardiac tolerance to global ischaemia. Acta cardio. 2007;62:381–389. [PubMed] [Google Scholar]
- Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sciences. 2016;152:244–248. [PubMed] [Google Scholar]
- Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–5175. [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78:77–87. [PMC free article] [PubMed] [Google Scholar]
- Klassen TL, Bomben VC, Patel A, Drabek J, Chen TT, Gu W, Zhang F, Chapman K, Lupski JR, Noebels JL, Goldman AM. High-resolution genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile. Epilepsia. 2014;55:e6–12. [PMC free article] [PubMed] [Google Scholar]
- Krebs P, Fan W, Chen YH, Tobita K, Downes MR, Wood MR, Sun L, Li X, Xia Y, Ding N, Spaeth JM, Moresco EM, Boyer TG, Lo CW, Yen J, Evans RM, Beutler B. Lethal mitochondrial cardiomyopathy in a hypomorphic Med30 mouse mutant is ameliorated by ketogenic diet. Proc Natl Acad Sci U S A. 2011;108:19678–19682. [PMC free article] [PubMed] [Google Scholar]
- Maguire MJ, Jackson CF, Marson AG, Nolan SJ. Treatments for the prevention of Sudden Unexpected Death in Epilepsy (SUDEP) The Cochrane database of systematic reviews. 2016;7:CD011792. [PMC free article] [PubMed] [Google Scholar]
- McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:7–11. [PMC free article] [PubMed] [Google Scholar]
- Moore BM, Jerry Jou C, Tatalovic M, Kaufman ES, Kline DD, Kunze DL. The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP) Epilepsia. 2014;55:1808–1816. [PubMed] [Google Scholar]
- Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet. 1999;353:888–893. [PubMed] [Google Scholar]
- Ozdemir R, Kucuk M, Guzel O, Karadeniz C, Yilmaz U, Mese T. Does ketogenic diet have any negative effect on cardiac systolic and diastolic functions in children with intractable epilepsy?: One-year follow-up results. Brain & development. 2016;38:842–847. [PubMed] [Google Scholar]
- Rho JM, Szot P, Tempel BL, Schwartzkrroin PA. Developmental seizure susceptibility of kv1.1 potassium channel knockout mice. Dev Neurosci. 1999;21:320–327. [PubMed] [Google Scholar]
- Schoeler NE, Cross JH, Sander JW, Sisodiya SM. Can we predict a favourable response to Ketogenic Diet Therapies for drug-resistant epilpesy? Epilepsy research. 2013;106:1–16. [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. [PMC free article] [PubMed] [Google Scholar]
- Simeone KA, Matthews SA, Rho JM, Simeone TA. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 2016;57:178–182. [PMC free article] [PubMed] [Google Scholar]
- Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 2009;5:492–504. [PubMed] [Google Scholar]
- Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB life. 2017;69:305–314. [PubMed] [Google Scholar]
- Zhao M, Huang X, Cheng X, Lin X, Zhao T, Wu L, Yu X, Wu K, Fan M, Zhu L. Ketogenic diet improves the spatial memory impairment caused by exposure to hypobaric hypoxia through increased acetylation of histones in rats. PloS One. 2017;12 0174477. [PMC free article] [PubMed] [Google Scholar]