- Journal List
- HHS Author Manuscripts
- PMC7359863

The Mitochondria-Targeted Antioxidant MitoQ Inhibits Memory Loss, Neuropathology, and Extends Lifespan in Aged 3xTg-AD Mice
Melissa L. Young
aThe University of Georgia College of Pharmacy, Department of Pharmaceutical and Biomedical Sciences, 357 Wilson Pharmacy, Athens, Georgia USA 30602
James L. Franklin
bThe University of Georgia College of Pharmacy, Department of Pharmaceutical and Biomedical Sciences, 357 Wilson Pharmacy, Athens, Georgia USA 30602
Abstract
Oxidative stress, likely stemming from dysfunctional mitochondria, occurs before major cognitive deficits and neuropathologies become apparent in Alzheimer’s disease (AD) patients and in mouse models of the disease. We previously reported that treating 2- to 7-month-old 3xTg-AD mice with the mitochondria-targeted antioxidant MitoQ (mitoquinone mesylate: [10-(4,5-Dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl](triphenyl)phosphonium methanesulfonate), a period when AD-like pathologies first manifest in them, prevents AD-like symptoms from developing. To elucidate further a role for mitochondria-derived oxidative stress in AD progression, we examined the ability of MitoQ to inhibit AD-like pathologies in these mice at an age in which cognitive and neuropathological symptoms have fully developed. 3xTg-AD female mice received MitoQ in their drinking water for five months beginning at twelve months after birth. Untreated 18-month-old 3xTg-AD mice exhibited significant learning deficits and extensive AD-like neuropathologies. MitoQ-treated mice showed improved memory retention compared to untreated 3xTg-AD mice as well as reduced brain oxidative stress, synapse loss, astrogliosis, microglial cell proliferation, Aβ accumulation, caspase activation, and tau hyperphosphorylation. Additionally, MitoQ treatment significantly increased the abbreviated lifespan of the 3xTg-AD mice. These findings support a role for the involvement of mitochondria-derived oxidative stress in the etiology of AD and suggest that mitochondria-targeted antioxidants may lessen symptoms in AD patients.
1. Introduction
Alzheimer’s disease is a neurodegenerative disorder characterized by increasing cognitive impairment and dementia coincident with progressive damage to brain neuronal tissue. Neuropathological hallmarks of the disease include the development of extracellular amyloid plaques in the brain and the formation of neurofibrillary tangles (NFT) within neurons. The plaques are primarily composed of amyloid β (Aβ) peptides cleaved from the amyloid precursor protein (APP; O’Brien and Wong, 2011) and the tangles of hyperphosphorylated tau, a cytoskeleton-associated protein (Weingarten et al., 1975; Delacourte et al., 1999; Frost et al., 2015). Oxidative stress occurs early in the development of AD, preceding development of most plaques and tangles and continuing throughout disease progression. Considerable evidence suggests that this stress may be a key mediator of the disease (Gutteridge, 1994; Praticò et al., 2002; Pope et al., 2008; Praticò, 2008; Moreira et al., 2010; Federico et al., 2012; Castellani et al., 2016).
A primary source of oxidative stress are reactive oxygen species (ROS) derived from the mitochondria electron transport chain (ETC; Nicholls and Ferguson, 2013; Halliwell and Gutteridge, 2015). Production of ROS by mitochondria occurs when electrons leak from the ETC to reduce dioxygen to the free radical superoxide (O2.−). The O2.− converts to additional ROS and other reactive species (RS). MitoQ is a mitochondria-targeted antioxidant composed of ubiquinone, a component of the ETC, covalently bound via a ten carbon chain to triphenylphosphonium (TPP+), a lipophilic cation that targets the ubiquinone moiety to the inner mitochondrial membrane driven by the high electrochemical potential across that membrane (Kelso et al., 2001; Smith et al., 2003). TPP+ remains on the matrix side of the membrane, allowing the ubiquinone to penetrate the membrane where respiratory complex II reduces it to ubiquinol. MitoQ acts as an antioxidant when oxidized back to ubiquinone by various RS (James et al., 2005; Smith and Murphy, 2010). Complex II reduces the ubiquinone to repeat the cycle. Because MitoQ is a poor substrate for complex I and III, it cannot substitute for endogenous ubiquinone and thus does not take part in mitochondrial respiration (James et al., 2005). It instead acts as a renewable antioxidant. MitoQ can cross the blood-brain barrier and, following a Nernstian distribution driven by the high potential across the inner mitochondrial membrane, concentrates hundreds of fold in mitochondria as compared to serum concentrations (Murphy and Smith, 2007). Ad libitum exposure to MitoQ in drinking water results in establishment of a steady-state concentration across this membrane within a few days (Smith et al., 2003). MitoQ is excreted unchanged or with sulfation and glucuronidation in the urine and bile (Li et al., 2007). Long-term ad libitum water administration of MitoQ to mice causes no major changes in physical activity, oxygen consumption, food consumption, body weight, or a number of other physical parameters (Rodriguez-Cuenca et al., 2009).
3xTg-AD mice express three mutant human transgenes, two that cause early-onset AD (APPswe and PS1M146V) and one that causes frontotemporal dementia (tauP301L; Oddo et al., 2003). While all mouse models of AD have limitations (Li et al., 2016), 3xTg-AD mice are perhaps the best for investigating development of AD as AD-like pathologies appear in them in an age-dependent sequence comparable to that seen in humans. Cognitive deficits occur as early as four months of age (Billings et al., 2005). Mitochondrial dysfunction and oxidative stress precede these deficits, comprising some of the earliest pathological signs noted in these mice (Resende et al., 2008; Yao et al., 2009). By six months of age, the presence of synaptic dysfunction and extracellular plaques are detectable. Tau pathology, present in this model as NFT, appears at about 12 months of age (Oddo et al., 2003). We previously evaluated the effect of MitoQ treatment on cognitive performance and neuropathology in young 3xTg-AD mice (McManus et al., 2011). In that study, we exposed 3xTg-AD mice to MitoQ in their drinking water for five months beginning at two months of age. This treatment prevented cognitive decline and associated AD-like pathologies.
To further assess the role that oxidative damage has in disease progression, we treated female 3xTg-AD mice with MitoQ after AD-like pathology had developed. Beginning at 12 months of age and continuing for five months, mice received MitoQ in all drinking water. This treatment significantly improved spatial memory retention and inhibited brain oxidative stress, astrogliosis, microglia cell proliferation, amyloid plaque formation, Aβ accumulation, tau hyperphosphorylation, and formation of NFT. MitoQ-treatment also increased 3xTg-AD lifespan, suggesting that the neuropathology ameliorated by MitoQ treatment is responsible for the accelerated death rate of these mice.
2. Material and methods
2.1. Reagents
Mitoquinone mesylate ([10-(4,5-Dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl](triphenyl)phosphonium methanesulfonate) complexed to β-cyclodextrin was a gift from Michael P. Murphy via GlycoSyn Technologies. We purchased all other reagents from Sigma-Aldrich unless otherwise noted.
2.2. Mice
The 3xTg-AD mouse model used in this study expresses three mutant human transgenes: amyloid precursor protein (APPswe), presenilin-1 (PS1M146V), and four-repeat tau (tauP301L; Oddo et al., 2003). Both of the mutations in amyloid precursor protein and presenilin-1 are associated with early-onset familial forms of AD and the tau mutation with frontotemporal dementia. AD-like symptoms of the disease appear in these mice as early as three-four months of age and continue to progress with time. We obtained founding 3xTg-AD breeders and control mice with the same 129/C57BL/6 hybrid genetic background but lacking the transgenes from The Jackson Laboratory. The study used only female 3xTg-AD mice and control mice because female 3xTg-AD mice develop greater Aβ burden than male mice (Carroll et al., 2010). The Jackson Laboratory also indicates that the female AD phenotype is more consistent than the male one. Beginning at 12 months of age and continuing for five months, female mice 3xTg-AD mice were administered 100 μM MitoQ complexed to β-cyclodextrin (1:4 ratio) in all drinking water. β-cyclodextrin, a cyclic oligosaccharide, was included to aid in solubilizing MitoQ, a lipophilic compound. Due to rapid degradation in the intestine and the ability of intestinal sugar transporters to transport only monosaccharides, β-cyclodextrin is not absorbed into the blood stream (Shimpi et al, 2005). Control animals did not receive β-cyclodextrin in their water as the quantity used to solubilize the MitoQ led to daily mouse consumption that was well below that known to have detectable effects on mouse plasma lipids levels (Wagner et al., 2008). Littermate controls and nonTg controls with the same 129/C57BL6 genetic background had access to drinking water that did not contain MitoQ. All mice were group housed in our animal facilities, given access to the same rodent chow, Bed-o’Cobs bedding (Andersons Lab), and were maintained on a 12 h light/dark cycle. All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3. Spatial learning and memory retention
Following the five-month treatment period, mice were assessed for spatial memory retention using the Morris Water Maze (MWM; Morris, 1984). For eight consecutive days, mice underwent acquisition trials in which they were trained to find and escape onto a hidden platform within the water maze. The water maze consisted of a circular aluminum tank (4ft diameter) filled with opaque water and one slightly submerged plexiglass platform, 14 cm in diameter. Water, made opaque with nontoxic white tempura paint, was maintained at 24 ± 1°C. Behavioral assessments were conducted as previously described with minor changes (McManus et al., 2011). Mice were placed on the hidden platform before the first acquisition trial for 10 s to reduce stress and establish existence of an escape platform. Acquisition trials followed in which mice were placed in the water maze at one of four predetermined starting points and allowed a 60 second free swim to escape onto the platform. Mice unable to find the platform were manually guided there and allowed 30 s on the platform to become familiar with distinct spatial cues in the test area. Each mouse underwent four trials each day with a 30 s rest on a warm towel between trials. Trials continued until mice met escape latency criterion defined as reaching the escape platform within 20 s or less. Spatial bias was determined in probe trials 1.5 and 24 h after the last acquisition trial. The platform was then removed and mice allowed a 60 s free swim. The time spent in the quadrant where the platform had been previously located was determined. To account for possible sensorimotor deficits, mice were subjected to a cued acquisition trial following the last probe trial. In the cued trials, the platform was replaced in the pool and visibly marked with a flag. Mice were placed in the maze at a novel position and allowed to find the newly placed platform. Times to reach the platform and swim speed were determined. Each mouse was handled and assessed for general health prior to cognitive assessment. All trials were recorded and analyzed using Ethovision Tracking software (Noldus Inc.) and SigmaPlot 11.1 (Systat Software).
2.4. Tissue acquisition
Mice were sacrificed in accordance with our animal use protocol with carbon dioxide followed by cervical dislocation. Brains were rapidly removed and split sagittally. Each cerebral hemisphere was either fixed in 4% paraformaldehyde for immunohistochemistry or used fresh or snap-frozen and stored at −80°C for immunoblotting or biochemical assays.
2.5. Immunoblotting
Harvested brain tissues were homogenized in radio immunoprecipitation assay (RIPA) buffer (50 mM Tris, 0.5% Sodium deoxycholate, 1% Triton X-100, 150 mM NaCl) supplemented with a protease inhibitor cocktail (Sigma-Aldrich Cat# P2714). Samples were centrifuged at 13,000 rcf at 4°C for 15 m using an Eppendorf 5417R centrifuge. Equal amounts of protein were separated via SDS-PAGE, and transferred to PVDF (Millipore) membranes for 1 hour in cold transfer buffer. The membranes were blocked with 5% non-fat milk in TBS-T (10 mM Tris-HCL, 100 mM NaCl, and 0.1% Tween-20) for 30 minutes at room temperature. Afterward, membranes were incubated with primary antibodies against glial fibrillary acid protein (GFAP) at 1:1000 (Thermo Fisher Cat# PA3–16727, RRID: AB_2109795), anti-synaptophysin at 1:1000 (Millipore Cat# MAB525820UG, RRID: AB_11214133), anti-nitrotyrosine at 1:1000 (Invitrogen Cat# ab61392, RRID: AB_942087), or anti-tau 5 at 1:500 (Santa Cruz Cat# sc-58860, RRID: AB_785931) at 4°C overnight. Membranes were then washed for at least 20 m in TBS-T and incubated at room temperature in an anti-mouse or anti-rabbit HRP-linked secondary antibody at 1:1000 (Cell Signaling Cat# 7076 and 7074, RRID: AB_330924 AB_2099233) followed by another 20 m wash. Anti-β-actin at 1:300 (GenScript Cat# A00730–40, RRID: AB_914100), and anti-β-tubulin at 1:1000 (Thermo Cat# PA1–41331, RRID: AB_2210397) were used for loading controls. Proteins were detected using Chemiluminescent ECL Western Blotting Substrate (Pierce).
2.6. Immunohistochemistry
Individual cerebral hemispheres were fixed for 48 h in 4% paraformaldehyde, embedded in paraffin, cut into 12 um sections, and mounted on glass slides. Sections were deparaffinized and rehydrated through a series of incubations in xylene and ethanol. Following rehydration, antigen retrieval was achieved with heated 10 mM sodium citrate buffer pH 6.0 at 95°C for 10 m in a humidity chamber. Antigen-retrieved sections were then incubated for 30 m in 0.3% H2O2 in MeOH and blocked with Vectastain Universal blocking serum (Vector Laboratories) at room temperature for 30 m. Following blocking, sections were incubated with anti-AT8 (Thermo Cat# MN1020, RRID:AB_223647), anti- tau5 (Santa Cruz Cat# sc-58860, AB_785931), and anti-AB42 (Bioss Inc. Cat# bs-0107R, RRID:AB_10858046) overnight at 4°C. Sections were visualized using an ABC immunoperoxidase kit from Vector Laboratories with diaminobenzidine substrate.
2.7. Amyloid β(1–42) ELISA
Soluble and insoluble fractions of Aβ(1–42) were detected in both whole brain tissue and hippocampal tissue with a BetaMark Colorimetric ELISA kit (Covance). Briefly, tissue was homogenized in ice cold 0.6% SDS lysis buffer (50 mM Tris, 2 mM EDTA, 150 mM NaCl) supplemented with a protease inhibitor cocktail (Sigma-Aldrich Cat# P2714). Samples were centrifuged at 25,000 rcf at 4°C for 1 h. Supernatant containing soluble amyloid or amyloid peptide standards were added to ELISA plates in duplicate and incubated overnight at 4°C. The following day the 96-well plate was thoroughly washed, incubated with tetramethylbenzidine substrate for 50 m at room temperature while protected from light, and the colorimetric product was determined by absorbance at 620 nm with a SpectraMax M2 microplate. The Bradford assay (Pierce) was utilized to ensure equal loading of protein.
2.8. Caspase 3/7 activity
Caspase 3/7 activity was measured using a Caspase-Glo 3/7 kit (Promega) following the manufacturer’s instructions. Briefly, brain tissue from each treatment group was homogenized in ice-cold hypotonic extraction buffer (25 mM HEPES, pH 7.5, 5 mM MgCl2, 1 mM EGTA) and centrifuged at 13,000 rpm for 15 minutes at 4°C. Protein concentrations were determined with the Bradford Assay (Pierce) and then diluted with PBS to achieve equal protein loading. Samples were incubated in a white-walled, clear-bottom 96-well plate for 1 hour with equal volume Caspase-Glo reagent. Luminescence (relative light units) was measured by a SpectraMax M2 microplate reader.
2.9. Statistics
Graphical representations were made and statistical significance measures determined with SigmaPlot 11.1 (Systat Software). Appropriate statistical analyses were conducted for experiments based on data distribution. Statistical comparisons were made via one-way ANOVA on Ranks with Dunn’s or Holm-Sidak post-hoc tests or by repeated measures ANOVA. Error bars represent ± SEM.
3. Results
3.1. MitoQ treatment enhanced cognitive performance and decreased synapse loss in aged 3xTg-AD mice
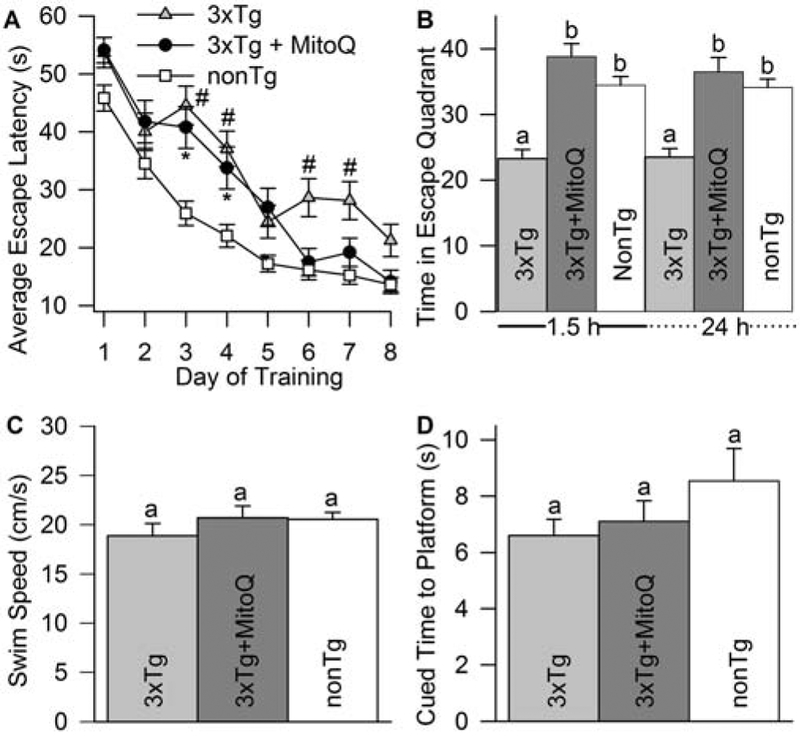
3xTg-AD mice were supplied with 100 μM MitoQ in their drinking water beginning at 12 months after birth and continuing until the18th month. At the end of the treatment period, spatial learning and memory retention were assessed with the MWM (Morris, 1984). All mice were able to achieve a baseline criterion defined as finding and escaping to the MWM platform in 20 s or less during the training trials (Fig 1A). Eighteen-month-old, MitoQ-treated 3xTg-AD mice reached criterion two days before age-matched littermate 3xTg-AD controls. Thus, MitoQ treatment significantly improved performance during acquisition trials.
MitoQ treatment improved spatial memory retention in 18-month-old female 3xTg-AD mice. A, Time courses of MWM spatial learning and memory acquisition in 18-month-old mice. All groups were able to perform the task with similar escape latencies after 8 d of training. However, MitoQ-treated mice reached criterion escape latency (< 20 s) 2 days earlier than 3xTg-AD mice (p < 0.01 for #3xTg and *3xTg + MitoQ as compared to nonTg by repeated measures ANOVA). B, MitoQ-treated mice showed significant improvement in both short- and long-term spatial memory retention compared to 3xTg-AD mice in probe trials conducted 1.5 and 24 h after the last training trial (p < 0.01). C, Swim speeds within the 18-month-old treatment groups were not significantly different, indicating that all mice had comparable sensorimotor capabilities (p = 0.664). D, Escape latency to the visible platform. All mice, despite treatment group, were capable of reaching the platform in the same amount of time (p = 0.107). Different letters in this and subsequent figures indicate significant differences while similar letters indicate no statistical difference (n = 15 3xTg-AD mice and 20 mice each for 3xTg-AD + MitoQ and nonTg).
To determine the effect of MitoQ treatment on memory retention, the escape platform was removed and mice were allowed a 60 s free swim. Spatial bias was measured by the amount of time the animals spent looking for the platform in the quadrant where the platform was previously positioned. Memory retention was determined at 1.5 and 24 h after the last training trial. The nonTg mice had significantly better memory retention at both time points as compared to 3xTg-AD mice. MitoQ increased retention in the 3xTg-AD mice to that of the nonTg mice at both time points (Fig. 1B). All mice were evaluated prior to the start of the training trials for general health. Swim speed was measured in the first trial. There were no discernable differences between the treatment groups, indicating that the mice had equal ability to reach the platform (Fig. 1C). Following the last acquisition trial, mice were subjected to a cued trial where the platform was placed back into the MWM at a visible novel location marked with a flag. All mice escaped the MWM to the cued platform within the same period. No significant differences were observed among groups, indicating that genotype or MitoQ treatment had no effect on sensorimotor capabilities (Fig. 1D; p = 0.107).
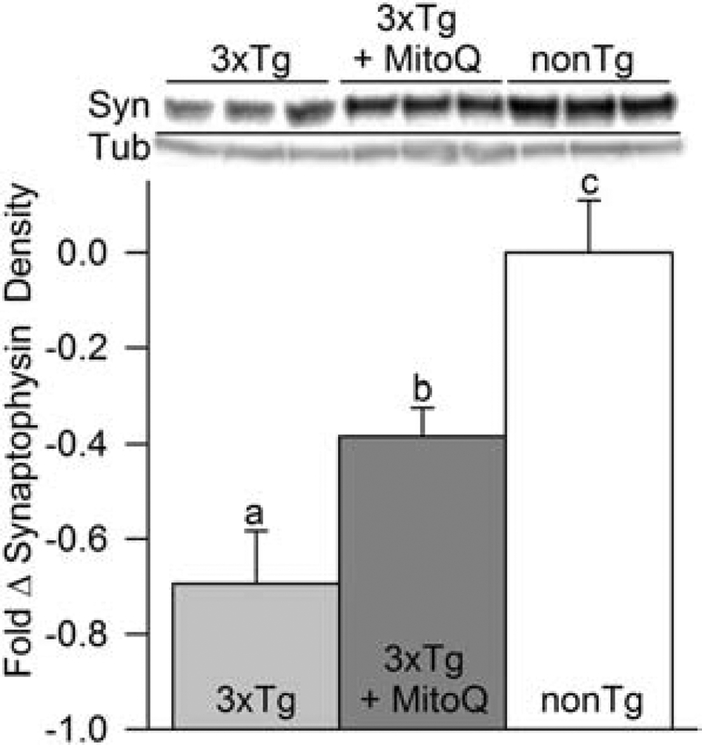
Synaptic dysfunction is a likely contributing factor causing the cognitive deficits in 3xTg-AD mice. There is a marked increase in synaptic dysfunction with increasing age in these mice coincident with a significant decline of the presynaptic vesicle glycoprotein synaptophysin in their brains (Oddo et al., 2003; McManus et al., 2011). MitoQ treatment of young 3xTg-AD mice inhibits synaptic loss concurrent with MitoQ-mediated inhibition of spatial memory retention deficits (McManus et al., 2011). Our behavioral data with the aged MitoQ-treated animals suggested that this treatment may have a protective effect on synaptic function in aged 3xTg-AD mice, as well. We used immunoblotting to quantify synaptophysin levels in cortical tissues of 18-month-old female nonTg, 3xTg-AD, and 3xTg-AD mice that had received MitoQ treatments for the preceding five months. The 3xTg-AD mice had much lower levels of synaptophysin than nonTg animals (Fig. 2). MitoQ treatment significantly inhibited synaptophysin loss in these animals, suggesting that preservation of synapses could be behind the improved cognitive performance of the MitoQ-treated mice.
MitoQ treatment protected against synapse loss in the brains of aged female 3xTg-AD mice. Synaptophysin (Syn) density was measured in immunoblots from homogenized cortical tissue. MitoQ-treated 3xTg-AD mice showed a significant reduction in the loss of synaptophysin compared to littermate 3xTg-AD controls. However, synaptophysin was also significantly lower in MitoQ-treated 3xTg-AD mice compared to age-matched nonTg mice, suggesting treatment simply slowed or halted further synapse loss. In this and subsequent immunoblot figures, density data was first normalized to the appropriate tubulin loading control and then to the average value of the similarly normalized nonTg controls. Fold change in all figures is shown as band density/control band density-1 (p < 0.01; n = 6 for each condition).
3.2. MitoQ treatment inhibited oxidative stress in 3xTg-AD mouse brains
Increased oxidative stress, a consequence of the overproduction and/or reduced clearance of RS, precedes most other neuropathologies associated with AD (Nunomura et al., 2001, 2006; Praticò et al., 2002; Castellani et al., 2016). MitoQ’s antioxidant moiety, ubiquinone, is reduced by complex II into ubiquinol, returning ROS-forming electrons to the ETC and regenerating the antioxidant ubiquinol moiety (Frei et al., 1990; James et al., 2005; Smith and Murphy, 2010).
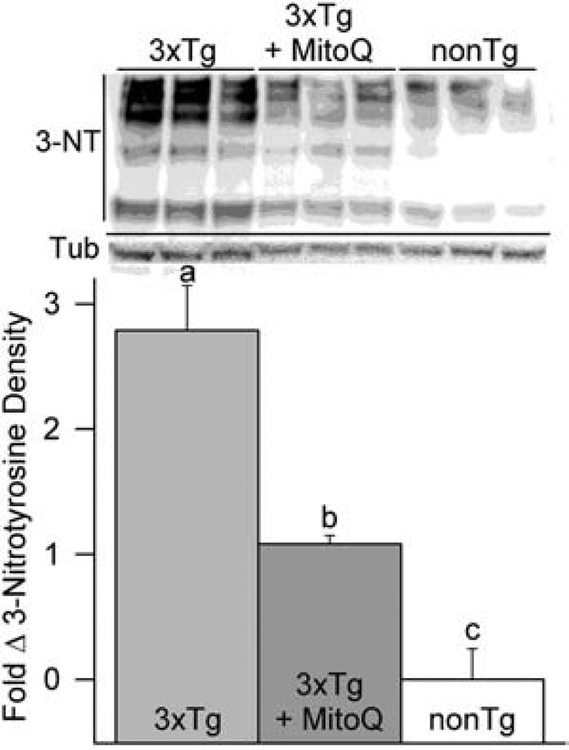
Oxidative stress can cause damage to DNA and proteins. Superoxide reacts with nitric oxide (NO) to produce peroxynitrite (ONOO−), a RS that can cause oxidative modifications to tyrosine residues in proteins. Nitrated modifications to proteins are elevated in humans with AD and in mouse models of AD (Smith et al., 1997; McManus et al., 2011). Our previous studies revealed that MitoQ attenuated ONOO−-associated reactive species in cortical neurons in vitro and decreased nitrated modification of proteins in the brains of young female 3xTg-AD mice (McManus et al., 2011). To determine MitoQ’s effect on nitrated modifications in late stage AD-like pathology, we measured nitrotyrosine levels in 18-month-old female mouse brain tissue with immunoblots (Fig. 3). Nitrotyrosine levels were greatly elevated in the 3xTg-AD brains as compared to that in the brains of nonTg animals. While MitoQ did not eliminate the presence of nitrated products, it did significantly reduce the presence of nitrotryosine compared to non-treated 3xTg-AD mice.
Accumulation of nitrated proteins, an oxidative stress marker, in the brains of 18-month-old female 3xTg-AD mice that had received five months of MitoQ treatment. MitoQ treatment reduced the buildup of nitrated tyrosine products. Decrease of the nitration marker 3-nitrotyrosine (3-NT) in the brains of the MitoQ-treated mice indicated that MitoQ inhibited accumulation of nitration produced in them. The bands are individual proteins that had been modified by nitration. Immunoblot densities are shown as fold change relative to average density in nonTg brains (p < 0.01; n = 3 for each condition).
3.3. MitoQ treatment inhibited astrogliosis and microglial cell proliferation
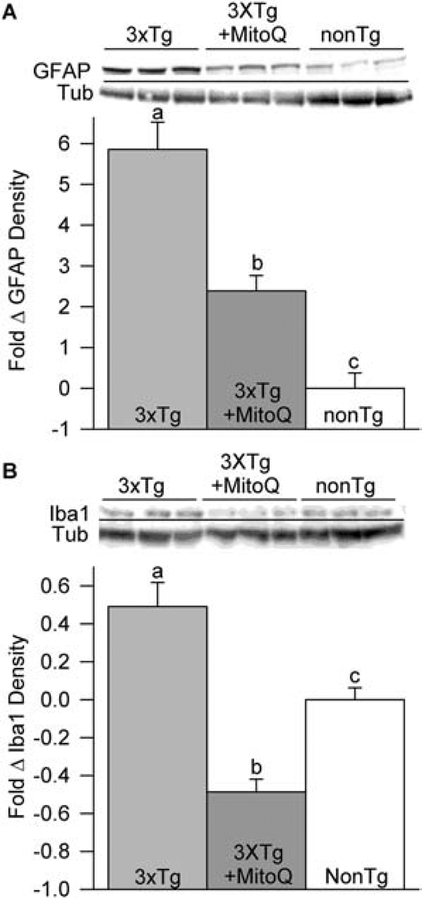
Increased numbers of reactive astrocytes (astrogliosis) and microglia cells, indicative of injury and inflammation, are found in postmortem brain tissue of human AD patients and in the brains of mouse models of AD (Oddo et al., 2003; Janelsins et al., 2005; McManus et al., 2011; Hansen et al., 2018). In our previous work, we showed that MitoQ treatment inhibited astrogliosis in young 3xTg-AD mice (McManus et al., 2011). To evaluate the effect MitoQ had on astrogliosis in late stage AD-like pathology, we used the astrocyte marker GFAP (Yang and Wang, 2015). MitoQ reduced GFAP band density in immunoblots of 3xTg-AD mouse brains by ~4-fold (Fig.4A) indicating it decreased astrocyte proliferation. Immunoblots of ionized calcium binding adaptor molecule 1 (Iba1), a microglial/macrophage marker (Imai et al., 1996), were elevated in the 3xTg-AD brains compared to nonTg controls. Figure 4B shows that Iba1 levels in brains from MitoQ-treated 3xTg-AD animals were significantly lower than Iba1 levels in the brains of both untreated nonTg animals and the brains of MitoQ-treated 3xTg-AD mice indicating significant inhibition of microglia cell proliferation by MitoQ.
MitoQ inhibited astrogliosis and microglial cell proliferation in the brains of aged female 3xTg-AD mice. A, Glial fibrillary acidic protein (GFAP), an astrocyte marker, was elevated in the brains of untreated 3xTg-AD mice compared to the brains of nonTg animals indicating proliferation of astrocytes. MitoQ treatment reduced GFAP levels to well below those found in 3xTg-AD mice, indicating that it greatly reduced astrocyte proliferation (p < 0.01; n = 3 for each condition). B, Iba1, a microglia cell marker, was elevated in the brains of 3xTg-AD mice compared to the brains of nonTg controls. MitoQ treatment reduced brain Iba1 levels to below those of both 3xTg-AD and nonTg animals, indicating significant reduction of microglia cell proliferation (p < 0.01; n = 4 for each condition).
3.4. MitoQ decreased amyloid burden in aged 3xTg-AD brains
Considerable evidence supports the existence of a positive feedback loop between Aβ deposition and oxidative stress. Introduction of pro-oxidants into neuronal cultures promotes the production of Aβ and stimulates signaling pathways that contribute to increased APP cleavage (Misonou et al., 2000; Tamagno et al., 2002, 2005, 2008; Quiroz-Baez et al., 2009). Specific interactions between Aβ and mitochondria can further propagate mitochondrial dysfunction and contribute to accelerated release of RS (Caspersen et al., 2005; Manczak et al., 2006; Mao et al., 2012). A direct correlation between oxidative stress and increased Aβ production in vivo has been demonstrated by introducing oxidizing agents directly into the hippocampus of wild-type mice, and observing a localized increase in Aβ deposition and pathological conformational changes associated APP cleavage enzymes (Arimon et al., 2015).
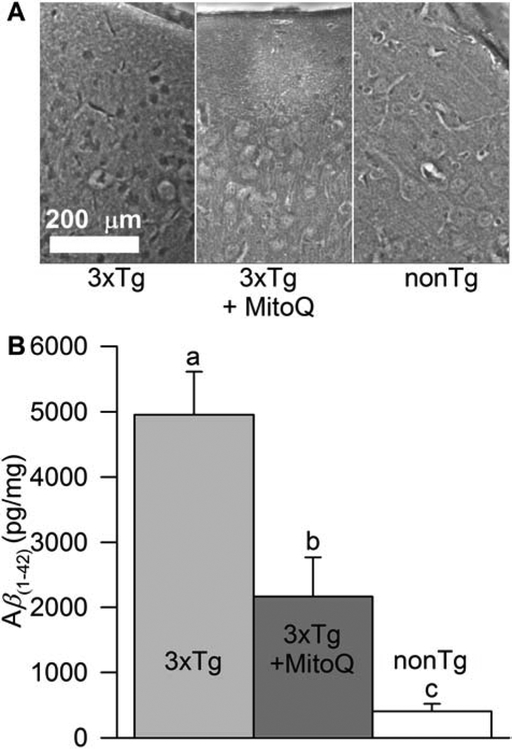
Intraneuronal Aβ staining is detectable in the brains of 3xTg-AD mice by four months after birth and appears extracellularly by six months (Oddo et al., 2003). By 12 months after birth, these pathologies are well established. Consistent with a role for RS in increasing brain Aβ levels, our previous work demonstrated that treating young 3xTg-AD mice for five months with MitoQ greatly inhibited the deposition of Aβ within their brains. To determine whether treatment of older animals with MitoQ would have a similar effect, we used immunohistochemistry and ELISA to evaluate Aβ(1–42) burden in the brains of untreated 3xTg-AD mice, 3xTg-AD mice treated with MitoQ for 5 months, and nonTg mice. Photomicrographs of cortical Aβ(1–42)-stained sections revealed intracellular and extracellular Aβ(1–42) deposition (Fig. 5A). Sections from nonTg mice showed little if any Aβ(1–42) staining, while those from MitoQ-treated 3xTg-AD animals had reduced staining compared to the untreated 3xTg-AD mice. Soluble Aβ(1–42) in the brains of 3xTg-AD mice was about 10-fold higher than in nonTg mice (Fig 5B). Five months of MitoQ treatment reduced this level by more than 2-fold to about 4-fold higher than that found in the brains of nonTg mice. The reduction in Aβ(1–42) deposition in the brains of 3xTg-AD mice by MitoQ treatment provides further evidence that increased mitochondria-derived RS modulates APP cleavage and increases Aβ deposition.
MitoQ decreased Aβ(1–42) burden in the brains of aged female 3xTg-AD mice. A, Representative photomicrographs showing immunostaining for Aβ(1–42) in the neocortex of 18-month-old female nonTg, 3xTg-AD, and 3xTg-AD mice that had received MitoQ treatment for the preceding five months. Intraneuronal and extracellular staining for Aβ(1–42) was largely absent in nonTg brains and reduced in brains from MitoQ-treated 3xTg-AD mice. B, Quantification of soluble Aβ(1–42) by ELISA revealed that MitoQ treatment significantly reduced amyloid burden in the 3xTg-AD mouse brain (p < 0.01; n = 6–8 for each condition).
3.5. MitoQ treatment decreased neurofibrillary tangles, total tau levels, phosphorylated tau levels, and caspase activity in the brains of 3xTg-AD mice
Tau pathology manifests in the 3xTg-AD mouse model in the form of NFT and is detectable in their brains by 12 months of age (Oddo et al., 2003). We used immunohistochemistry to investigate formation of NFT in the brains of 18-month-old female nonTg, 3xTg-AD, and 3xTg-AD mice that had received MitoQ treatment for the preceding five months. Figure 6A shows that MitoQ-treated 3xTg-AD mice had fewer NFT in their cortex than did untreated 3xTg-AD mice. No tangles were apparent in nonTg mice. We quantified total tau protein expression and tau phosphorylation levels by immunoblot. Total tau levels were significantly elevated in the brains of 3xTg-AD mice compared to nonTg ones. MitoQ-treated mice had total tau levels similar to those of nonTg mice, indicating that MitoQ decreased the build-up of tau in the brain (Fig 6B). Tau phosphorylation levels, occurring at AD-associated phosphorylation residues Ser202/205, were about 10-fold higher in immunoblot band densities in the 3xTg-AD brains than in the nonTg ones. MitoQ treatment reduced phosphorylated tau to a level that was indistinguishable from that of nonTg brains (Fig.6C).
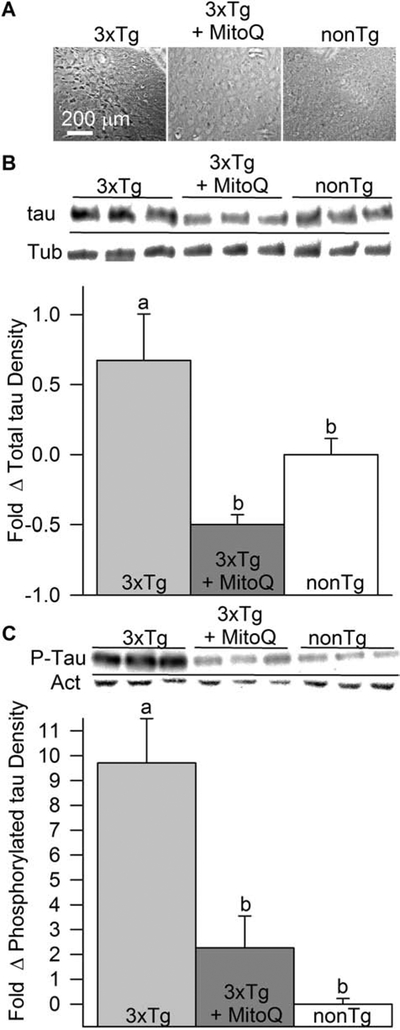
MitoQ decreased tau pathology in the brains of aged female3xTg-AD mice. A, Representative photomicrographs showing NFT in the neocortex of 18-month-old female nonTg, 3xTg-AD, and 3xTg-AD mice that had received MitoQ treatment for the preceding five months. Staining is for total tau protein. B, MitoQ treatment greatly decreased total tau levels in the brains of these mice as measured by immunoblots (p < 01; n = 3 for each condition). C. MitoQ treatment also greatly inhibited phosphorylated tau levels in the brains of these mice (p < 0.01; n = 3 for each condition).
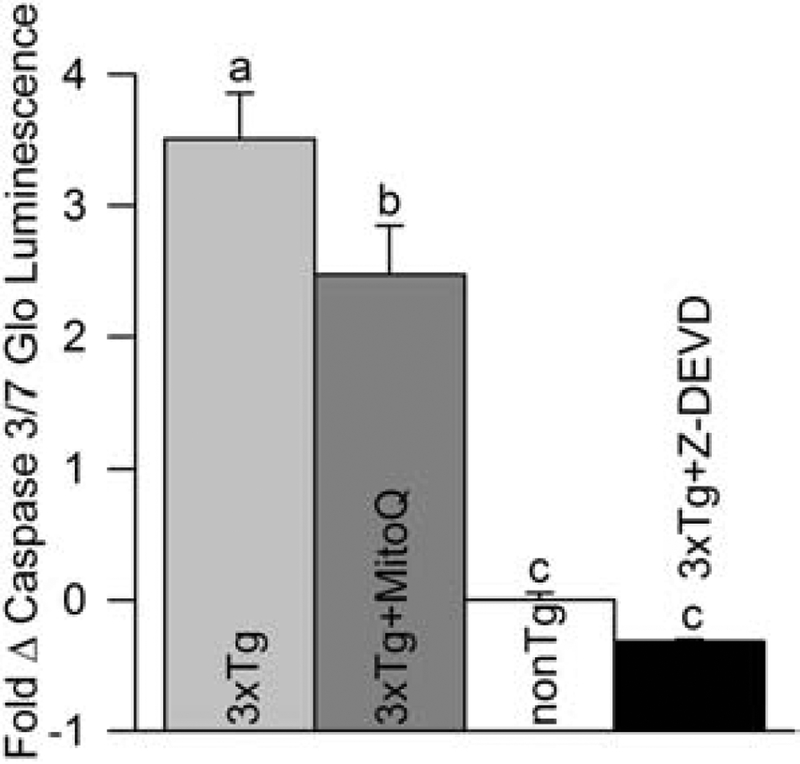
Caspase-mediated cleavage of tau is involved in the development of NFT (Rissman et al., 2004; Rohn et al., 2008; de Calignon et al., 2010). Given that stabilization of oxidative stress in young 3xTg-AD mice inhibits elevated caspase activity (McManus et al., 2011), we evaluated MitoQ’s effect on caspase activity in 18-month-old female 3xTg-AD mice that had received five months of MitoQ treatment. Caspase-3/7 activity was measured in brain tissue using a luminescent caspase activity assay. Capase 3/7 activity was elevated about 3.5-fold in the 3xTg-AD brains as compared to the nonTg ones. Relative luminescent values demonstrated that MitoQ treatment blocked caspase activation by about 20% relative to 3xTg-AD mice (Fig. 7).
MitoQ treatment reduced Caspase 3/7 activity in the brains of aged female 3xTg-AD mice. Caspase 3/7 activity in brain homogenates was analyzed with the Caspase-Glo 3/7 assay. Relative luminescence values, produced with a caspase 3/7-specific substrate, were measured by a luminometer. Values were normalized to nonTg caspase activity. Z-DEVD (50 μM), a broad-spectrum caspase inhibitor, reduced elevated caspase activity in 3xTg brain homogenates, confirming caspase activity was measured by the assay (p < 0.01; n = 6–10 except for Z-DEVD where n = 2).
3.6. MitoQ treatment increased 3xTg-AD mice lifespan
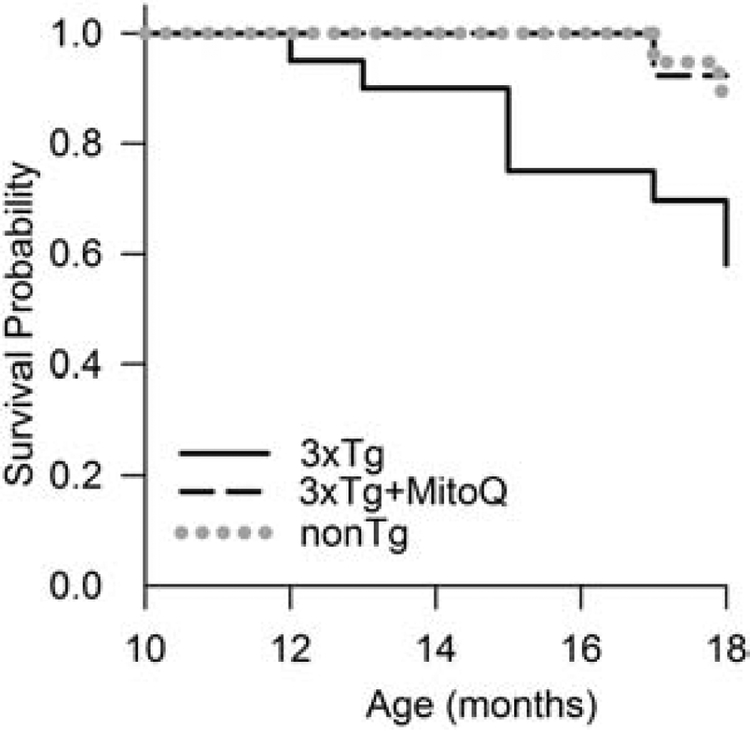
The median lifespan of 3xTg-AD mice is ~22 months with females living a bit longer than males (Rae and Brown, 2015). The median lifespan of nonTg mice with the same genetic background (129/C57BL/6) is ~34 months, also with females living a bit longer than males. Death of 3xTg-AD mice occurs as early as 12 months after birth. The common time frame of NFT development and the occurrence of mortality in the 3xTg-AD mouse model suggest that tau pathology may have a role in shortening the lifespan of these mice. As previously reported, we saw significant mortality in untreated 3xTg-AD mice by 12 months of age. MitoQ-treated mice had a lifespan that was indistinguishable from the nonTg mice within the 18-month period of the study (Fig. 8). These data show that, in addition to improved cognition, reduction of brain oxidative stress, and neuropathology, MitoQ extended the lifespan of this mouse model of AD.
Aged female 3xTg-AD mice died earlier than nonTg controls. MitoQ treatment increased the lifespan of these mice to that of the nonTg controls (p < 0.07 comparing MitoQ-treated to untreated 3xTg-AD mice). Survival was determined by Kaplan-Meier log-rank analysis for all mice until an end-point of 18 months of age (n = 15–20 mice).
4. Discussion
Evidence of oxidative stress is found in the brains of individuals who have died with AD and in the cerebrospinal fluid of patients with mild cognitive impairment, a condition that can be prodromal to AD (Nunomura et al., 2001, 2006; Praticò et al., 2002; Castellani et al., 2016). This stress precedes most of the neuropathological hallmarks of AD, suggesting that it may lie upstream from them. Mitochondrial dysfunction also occurs early in AD progression and is the likely source of the RS causing oxidative stress (Gutteridge, 1994; Pope et al., 2008; Moreira et al., 2010; Federico et al., 2012; Castellani et al., 2016). Oxidative stress and mitochondrial dysfunction are found not only in human AD but also in AD-like pathology in mouse models of the disease (Velliquette et al., 2005; Anantharaman et al., 2006; Butterfield et al., 2001, 2006, 2007; Resende et al., 2008; Yao et al., 2009; McManus et al., 2011).
The appearance of oxidative stress in the development of both human and mouse models of AD and the success of antioxidants in treating AD-like symptoms in preclinical animal trials has led to a number of human antioxidant clinical trials for treating AD (Persson et al., 2014). These trials have largely been unsuccessful (Castellani et al., 2016). Possible reasons for these failures include poor ability of the antioxidants to cross the blood-brain barrier, inability to reach and/or enter mitochondria in sufficient concentrations, treatment so late in the disease that irreparable damage has occurred, or that oxidative stress is merely associated with AD and is not critical for its etiology in humans as it appears to be in animal models. Several AD trials have used N-acetyl-L-cysteine (L-NAC), a compound that does not target mitochondria but acts as an antioxidant in part by increasing levels of glutathione, a major cellular antioxidant. A concentration of about 10 mM L-NAC is needed to block neuronal degeneration caused by exposing cultures of cortical neurons to Aβ (Hsiao et al., 2008). Similar concentrations of L-NAC are needed to block the apoptotic death of NGF-deprived sympathetic neurons in culture, a form apoptosis that appears to require increased mitochondrial production of ROS (Kirkland et al, 2001). MitoQ blocks this death at nM concentrations. A one nM concentration of MitoQ will block Aβ-induced death of cortical neurons in culture (McManus et al, 2011). This is a concentration seven orders of magnitude lower than the L-NAC concentrations required to do so. It is uncertain how much L-NAC can cross the human blood brain barrier and whether such concentrations can be reached in the brains of treated patients (Shahripour et al., 2014). Another antioxidant, vitamin E, has also had little success in several AD clinical trials (Persson et al., 2014). Like L-NAC, vitamin E does not target mitochondrial ROS production acting instead to target lipid peroxidation (Halliwell and Gutteridge, 2015) a form of oxidative damage lying downstream of mitochondrial ROS and that is largely independent of other ROS-induced damage such as DNA and protein oxidation. Problems with drug pharmacodynamics and pharmacokinetics are found with other antioxidants tested to date (Castellani et al., 2016). The development of mitochondria-targeted antioxidants, such as MitoQ, that cross the blood-brain barrier and concentrate in mitochondria, the principal source of most ROS, provide a better tool for determining whether mitochondria-associated oxidative stress is important for the disease and, perhaps, may lead to new and novel therapies for treating it (Zhao et al., 2004; James et al., 2005; Murphy and Smith, 2007; McManus et al., 2011).
We previously demonstrated the ability of MitoQ to inhibit cognitive decline and AD-like neuropathologies in young 3xTg-AD mice. That study focused on prevention of disease development (McManus et al., 2011). MitoQ treatment began two months after birth and continued for five months, a period during which the first AD-like pathologies become manifest. The current study focused not on disease prevention but on therapy for disease that had already become patent. We evaluated the effects of MitoQ treatment on cognitive decline and neuropathologies in 3xTg-AD mice starting at 12 months after birth and continuing until 18 months of age. During this period, all of the known AD-like pathologies are present and are progressing in severity (Oddo et al., 2003). As in the younger 3xTg-AD mice, we found that MitoQ treatment of older mice was effective in improving spatial memory retention. Levels of the synaptic protein synaptophysin were significantly higher in the brains of MitoQ-treated mice than in the brains of untreated ones indicating that MitoQ treatment inhibited synapse loss and suggesting that this inhibition might underlie the observed cognitive improvement.
Quantification of protein nitrotyrosine levels indicated that, at least by this measure, the brains of 18-month-old 3xTg-AD animals were under considerably greater oxidative stress than nonTg brains. MitoQ treatment greatly reduced nitration but not to the level found in nonTg animals. Overexpressing mitochondria superoxide dismutase in transgenic mouse models of AD decreases Aβ production and inhibits cognitive decline (Li et al., 2004; Esposito et al., 2006; Massad et al., 2009). Similarly, treating young 3xTg-AD mice with MitoQ prevents increased levels of Aβ in their brains (McManus et al., 2011). Aβ peptides are produced by sequential cleavage of APP by β- and γ-secretases. Oxidative stress can increase Aβ production by increasing β- and γ-secretase expression and activity via stress-activated kinases (Tong et al., 2005; Tamagno et al., 2002, 2005, 2008; Karupagounder et al., 2009; Zhang et al, 2011). Oxidative stress can also lead to modification of caspase activity (Circu and Aw, 2010), and activated caspases can cleave APP (Banwait et al., 2008; Bredesen et al., 2010; Mukherjee and Williams, 2017). Aβ has toxic effects on mitochondria (Lustbader et al., 2004; Sorrentino et al., 2018). Aβ can enter mitochondria via the TOM import machinery and induce mitochondrial dysfunction, at least in part, by inhibiting preprotein maturation (Petersen et al., 2008; Mossman et al., 2014). This inhibition imbalances the organelle proteome causing decreased respiration and increased ROS production. Therefore, increased Aβ in AD may involve a positive feedback cycle in which mitochondria-derived oxidative stress increases Aβ production, and the Aβ then increases ROS via its effects on mitochondria. Consistent with such a cycle, MitoQ decreased both oxidative stress and Aβ levels in the 18-month-old 3xTg-AD brains.
Increased numbers of astrocytes and microglial cells are found in both human AD brains and the brains of 3xTg-AD mice (Oddo et al., 2003; Janelsins et al., 2005; McManus et al., 2011; Hansen et al., 2018). It is uncertain as to whether these inflammatory processes contribute to AD or are downstream of the neuropathological processes. There is evidence that microglial cells are involved in the etiology of the disease, as well as evidence that they are protective against it (Hansen et al., 2018). The ability of MitoQ treatment to reduce Aβ levels, reactive astrogliosis, and microglial cell proliferation is consistent with the proliferation of both astrocytes and microglial cells lying downstream of the Aβ pathology.
The amyloid hypothesis postulates that Aβ deposition lies upstream of NFT formation (Hardy and Allsop, 1991). Aβ deposition occurs earlier in human AD and the 3xTg-AD mouse model of AD than do NFT (Oddo et al., 2003; Selkoe and Hardy, 2016). Many studies have confirmed the interdependence of Aβ and tau pathology (Sato et al., 2018). Mutations in human APP that increase amyloid deposition also lead to increased levels of tau in neurons (Bateman et al., 2012; Selkoe and Hardy, 2016). Introduction of Aβ into neuronal cultures can cause hyperphosphorylation of tau, necessary for formation of NFT (Jin et al., 2011). Experimental treatments that reduce Aβ burden also reduce NFT in 3xTg-AD mice (Oddo et al., 2004; Rasool et al., 2013; Dai et al., 2017). In the present study, MitoQ treatment reduced Aβ accumulation, NFT formation, and both total amounts of tau and hyperphosphorylated tau in the 3xTg-AD brains. Cleavage of tau by caspases can lead to tau aggregation and phosphorylation (Rissman et al., 2004; Rohn et al., 2008). Thus, MitoQ decreased two events thought to lie upstream of tau pathology, Aβ accumulation, and caspase 3 activity.
The increased lifespan in the MitoQ-treated 3xTg-AD mice was intriguing. The reason that these animals die earlier than nonTg ones has not been reported and we did not do necropsies of dead animals. However, the most likely explanation for the extended survival was the reduction in neuropathologies associated with the disease. A similar extension of lifespan by MitoQ treatment of a transgenic C. elegans strain that expresses high levels of Aβ indicates that excess Aβ levels can have deleterious effects cross-phyla and that targeting mitochondria can help alleviate these effects (Ng et al., 2014). Further investigation of the means of death of these mice and the effect of MitoQ treatment on mortality will be needed to determine the mechanism.
Comparing some of the parameters measured in our previous study of the effects of MitoQ on young animals to the effects of MitoQ on these parameters in the aged animals of this study revealed that MitoQ was highly effective in blocking progression of almost all AD-like symptoms in the young mice but merely inhibited their progression in the older animals (McManus et al., 2011). Synaptophysin (immunoblot band densities) in young 3xTg brains averaged about 60% of those from the nonTg brains. MitoQ treatment increased this to 100% of the nonTg level. The synaptophysin levels in the brains of the aged 3xTg-AD mice was lower than that in the brains of the younger animals with only about 30% of the synaptophysin level in the nonTg brains. MitoQ treatment increased this to about 60% of the nonTg brains. The 3xTg-AD nitrotyrosine levels in the brains of the young animals were about 3.8-fold higher than in the nonTg mouse brains as compared to about 2.8-fold higher in the brains of the older animals. MitoQ treatment decreased the nitrotyrosine band density to nonTg levels in the earlier study while, in the present study, nitrotyrosine levels decreased by about 64% but remained about 1-fold higher than in the nonTg brains. The 3xTg-AD GFAP in the original study was about 2.5-fold higher than that of the nonTg brains. MitoQ treatment reduced this to the nonTg level. In the current study, GFAP was about 6-fold higher than in the nonTg brains. MitoQ treatment reduced this to about 2-fold higher than nonTg levels. As have others (Belfiore et al., 2018), we found that the young 3xTg-AD mice in the early study had relatively low brain Aβ levels. The Aβ concentration increased by several orders of magnitude by 18 months of age. In the earlier study, MitoQ decreased Aβ burden from about 6.5 pg/mg total protein to about 1.8 pg/mg (72% decrease) as compared to the nonTg level of about 0.5 pg/mg. Nanogram levels of Aβ were found in the brains of the 18-month-old animals. We found about 5 ng/mg protein in the brains of the untreated 3xTg mice as compared to about 0.4 ng/mg protein in the nonTg animals. MitoQ treatment reduced the concentration to about 2 ng/mg protein (60% decrease). MitoQ treatment was far more effective in reducing caspase activity in the brains of the young mice (to control levels of nonTg animals) than in the aged ones where activity was reduced only by about 31%. Thus, the trend of the effect of MitoQ treatment on the investigated parameters was similar in the old as compared to the young mice but the degree of the effect was blunted. However, even though not as effective at ameliorating pathology in the old animals as the young ones, the data demonstrate considerable therapeutic efficacy in them suggesting the possibility that MitoQ might be useful in treating AD after it has become patent.
In summary, our findings show that the mitochondria-targeted antioxidant MitoQ reduced cognitive and AD-like neuropathological symptoms in old 3xTg-AD mice. In humans, amyloid plaques can occur in the absence of cognitive deficits, and there is a poor correlation between the plaques and neuropathology (Maarouf et al., 2011; Wirth et al., 2013; Altman et al., 2015). Those findings, combined with the plethora of failed clinical trials targeting Aβ (Hardy and De Strooper, 2017), lends credence to the idea that these pathologies may contribute to AD but are perhaps not the singular cause of the disease. Evidence that oxidative stress and mitochondrial dysfunction precedes most of the major AD pathologies and likely contributes to them suggests that targeting them may be more effective in treating the disease than targeting Aβ or tau. This idea is further supported by recent work showing that enhancing mitochondria proteostasis in an AD mouse model reduces Aβ-associated proteotoxic stress (Sorrentino et al., 2018). We, and others, have shown that antioxidants targeted directly to mitochondria, the source of most RS, are effective in reducing AD pathology in animal models of AD (Szeto, 2006; McManus et al., 2011; Mao et al., 2012). It should also be noted that while 3xTg-AD mice exhibit many of the symptoms seen in AD patients, they do not at any age show loss of neurons in the brain, a prominent feature of human AD (Virgili et al., 2018). Therefore, the positive effects on 3xTg-AD cognition by MitoQ that we report here were likely due solely to synapse preservation. At least some of the failure of AD drug trials may result from the fact that significant, irreversible brain damage with neuron loss has already occurred by the time the treatment started. Early detection and treatment before this has occurred is imperative. It would be of interest to determine whether MitoQ can prevent neuronal loss in an AD mouse model that does lose neurons, such as the 5xFAD model (Oakley et al., 2006).
The findings reported here and in our previous work (McManus et al., 2011) show that MitoQ decreases oxidative stress in the brains of a mouse model of AD and prevents or at least slows the progression of the disease in these animals. Clinical trials with MitoQ will be necessary to determine whether human AD is similarly affected by MitoQ treatment and may be able to answer the role, if any, of mitochondrial ROS production in the etiology of the disease.
Highlights
- Five months of treatment with MitoQ, a mitochondria-targeted antioxidant, inhibited loss of spatial memory retention in aged (12–18 month-old) 3xTg-AD mice.
- MitoQ also inhibited synapse loss, brain oxidative stress, reactive astrogliosis, microglial cell proliferation, brain Aβ deposition, and formation of neurofibrillary tangles in these mice.
- MitoQ extended the lifespan of the 3xTg-AD mice to that of control mice.
- These data support a role for mitochondria-produced reactive oxygen species in the development of Alzheimer’s-like pathology in this mouse model of the disease.
- We previously demonstrated that MitoQ prevents development of disease symptoms in young 3xTg-AD mice. The present data extends this finding and shows that MitoQ also has protective effects in aged mice that have already developed the disease.
Acknowledgments:
We would like to thank Rebecca A. Kirkland for editorial assitance. This work was supported by NIH grant R03 AG051205.
Abbreviations:
| AD | Alzheimer’s disease |
| NFT | neurofibrillary tangles |
| Aβ | amyloid |
| APP | amyloid precursor protein |
| ROS | reactive oxygen species |
| ETC | electron transport chain |
| O2.− | superoxide |
| RS | reactive species |
| TPP+ | triphenylphosphonium |
| MWM | Morris water maze |
| GFAP | glial fibrillary acid protein |
| NO | nitric oxide |
| ONOO− | peroxynitrite |
| Iba | ionized calcium binding adaptor molecule 1 |
| L-NAC | N-acetyl-L-cysteine |
| Syn | Synaptophysin |
| 3-NT | 3-nitrotyrosine |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Altmann A, Ng B, Landau SM, Jagust WJ, Greicius MD (2015) Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain 138: 3734–3746. [PMC free article] [PubMed] [Google Scholar]
- Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK (2006) β-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLh/NLh X PS-1 P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am J Pathol 168:1608–1618. [PMC free article] [PubMed] [Google Scholar]
- Arimon M, Takeda S, Post KL, Svirsky S, Hyman BT, Berezovska O (2015) Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis 84: 109–119. [PMC free article] [PubMed] [Google Scholar]
- Armstrong D, Browne R (1994) The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol 366: 43–58. [PubMed] [Google Scholar]
- Banwait S, Galvan V, Zhang J, Gorostiza OF, Ataie M, Huang W, Crippen D, Koo EH, Bredesen DE (2008) C-Terminal Cleavage of the Amyloid-β protein precursor at asp664: a switch associated with Alzheimer’s disease. J Alzheimers Dis 13: 1–16. [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, et al. (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367: 795–804 [PMC free article] [PubMed] [Google Scholar]
- Belfiore R, Rodin A, Ferreira E, Velazquez R, Branca C, Caccamo A, Oddo S (2018) Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell: 10.1111/acel.12873 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron 45: 675–688. [PubMed] [Google Scholar]
- Bredesen DE, John V, Galvan v (2010) Importance of the Caspase Cleavage Site in Amyloid-β Protein Precursor. J Alzheimers Dis 22: 57–63. [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid β-peptide. Trends Mol Med 7: 548–554. [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Sultana R (2006) Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol 545: 39–50. [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R (2007) Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med 43: 658–677. [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ (2010) Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res 1366: 233–245. [PMC free article] [PubMed] [Google Scholar]
- Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD (2005) Mitochondrial Aβ: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J 19: 2040–2041. [PubMed] [Google Scholar]
- Castellani RJ, Correia SC, Moreira PI, Perry G (2016) Tackling Alzheimer’s disease by targeting oxidative stress and mitochondria In: Developing therapeutics for Alzheimer’s disease (Wolfe MS, ed), pp 477–502. New York: Academic Press. [Google Scholar]
- Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems and apoptosis. Free Radic Biol Med 48: 749–762. [PMC free article] [PubMed] [Google Scholar]
- Dai CL, Tung YC, Liu F, Gong CX, Iqbal K (2017) Tau passive immunization inhibits not only tau but also Aβ pathology. Alzheimers Res Ther 9: 1. [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT (2010) Caspase activation precedes and leads to tangles. Nature 464: 1201–1205. [PMC free article] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C (1999) The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52: 1158–1165. [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puoliväli J, Scearce-Levie K, Masliah E, Mucke L (2006) Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci 26:5167–5179. [PMC free article] [PubMed] [Google Scholar]
- Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322: 254–262. [PubMed] [Google Scholar]
- Frei B, Kim MC, Ames BN (1990) Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci USA 87: 4879–4883. [PMC free article] [PubMed] [Google Scholar]
- Frost B, Götz J, Feany MB (2015) Connecting the dots between tau dysfunction and neurodegeneration. Trends Cell Biol 25: 46–53. [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JM (1994) Hydroxyl radicals, iron, oxidative stress, and neurodegeneration. Ann N Y Acad Sci 738: 201–213. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine, Ed 5 Oxford, UK: Oxford UP. [Google Scholar]
- Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217: 459–472. [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. TIPS 12: 383–388. [PubMed] [Google Scholar]
- Hardy J, De Strooper B (2017) Alzheimer’s disease: where next for anti-amyloid therapies? Brain 140: 853–855. [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S (1996) A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224: 855–862. [PubMed] [Google Scholar]
- James AM, Cocheme HM, Smith RA, Murphy MP (2005) Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem 280: 21295–21312. [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ (2005) Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J Neuroinflammation 2: 23. [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ (2011) Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA 108: 5819–5824. [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman D, Baker H, Beal MF, Gandy SE, Gibson GE (2009) Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiol Aging 30:1587–1600. [PMC free article] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596. [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Beal MF, Lin MT, Gouras GK (2004) Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem 89:1308–1312. [PubMed] [Google Scholar]
- Li Y, Zhang H, Fawcett JP, Tucker IG (2007) Quantitation and metabolism of mitoquinone, a mitochondria-targeted antioxidant, in rat by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21: 1958–1964. [PubMed] [Google Scholar]
- Li X, Bao X, Wang R (2016) Experiemental models of Alzheimer’s disease for deciphering the pathogeneis and therapeutic screening. Int J Mol Med 37:271–283. [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C,Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF,Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS,Wu H (2004) ABAD directly links Aβ to mitochondrial toxicity in Alzheimer’s disease. Science 304: 448–452. [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH (2006) Mitochondria are a direct site of Aβ accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15: 1437–1449. [PubMed] [Google Scholar]
- Maarouf CL, Daugs ID, Kokjohn TA, Walker DG, Hunter JM, Kruchowsky JC, Woltjer R, Kaye J, Castano EM, Sabbagh MN, Beach TG, Roher AE (2011) Alzheimer’s disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One 6: e27291. [PMC free article] [PubMed] [Google Scholar]
- Mao P, Manczak M, Calkins MJ, Truong Q, Reddy TP, Reddy AP, Shirendeb U, Lo HH, Rabinovitch PS, Reddy PH (2012) Mitochondria-targeted catalase reduces abnormal APP processing, amyloid β production and BACE1 in a mouse model of Alzheimer’s disease: implications for neuroprotection and lifespan extension. Hum Mol Genet 21: 2973–2990. [PMC free article] [PubMed] [Google Scholar]
- Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106:13576–13581. [PMC free article] [PubMed] [Google Scholar]
- McManus MJ, Murphy MP, Franklin JL (2011) The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci 31: 15703–15715. [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Morishima-Kawashima M, Ihara Y (2000) Oxidative stress induces intracellular accumulation of amyloid β-protein (Aβ) in human neuroblastoma cells. Biochemistry 39: 6951–6959. [PubMed] [Google Scholar]
- Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010) Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta 1802: 2–10. [PubMed] [Google Scholar]
- Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60. [PubMed] [Google Scholar]
- Mossmann D, Vögtle FN, Taskin AA, Teixeira PF, Ring J, Burkhart JM, Burger N, Pinho CM, Tadic J, Loreth D, Graff C, Metzger F, Sickmann A, Kretz O, Wiedemann N, Zahedi RP, Madeo F, Glaser E, Meisinger C (2014) Amyloid-β Peptide induces mitochondrial dysfunction by inhibition of preprotein maturation. Cell Metab 20: 662–669. [PubMed] [Google Scholar]
- Mukherjee A, Williams DW (2017) More alive than dead: non-apoptotic roles for caspases in neuronal development, plasticity and disease. Cell Death Differ 24: 1411–1421. [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Smith RA (2007) Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 47: 629–656. [PubMed] [Google Scholar]
- Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B (2014) The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med 71: 390–401. [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ (2013) Bioenergetics, Ed 4. London, UK: Academic Press. [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA (2001) Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 60: 759–67. [PubMed] [Google Scholar]
- Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA (2006) Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol 65: 631–41. [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26: 10129–40. [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34: 185–204. [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39: 409–421. [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM (2004) Aβ immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43: 321–332. [PubMed] [Google Scholar]
- Persson T, Popescu BO, Cedazo-Minguez A (2014) Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxid Med Cell Long: 427318. [PMC free article] [PubMed] [Google Scholar]
- Petersen CAH, Alikhani N, Behbahani H, Wiehager B, Pavlov BF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M (2008) The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci U SA 105: 13145–13150. [PMC free article] [PubMed] [Google Scholar]
- Pope S, Land JM, Heales SJ (2008) Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim Biophys Acta 1777: 794–799. [PubMed] [Google Scholar]
- Praticò D (2008) Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci 29: 609–615. [PubMed] [Google Scholar]
- Praticò D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ (2002) Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol 59: 972–976. [PubMed] [Google Scholar]
- Quiroz-Baez R, Rojas E, Arias C (2009) Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of α-, β- and γ-secretase expression. Neurochem Int 55: 662–670. [PubMed] [Google Scholar]
- Rae EA, Brown RE (2015) The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci Biobehav Rev 57: 238–251. [PubMed] [Google Scholar]
- Rasool S, Martinez-Coria H, Wu JW, LaFerla F, Glabe CG (2013) Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Aβ deposition and tau pathology in 3xTg-AD mice. J Neurochem 126: 473–482. [PMC free article] [PubMed] [Google Scholar]
- Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR (2008) Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med 44: 2051–2057. [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114: 121–130. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Cochemé HM, Logan A, Abakumova I, Prime TA, Rose C, Vidal-Puig A, Smith AC, Rubinsztein DC, Fearnley IM, Jones BA, Pope S, Heales SJR, Lam BYH, Neogi SG, McFarlane I, James AM, Smith RAJ, Murphy MP (2010) Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med 48: 161–172. [PubMed] [Google Scholar]
- Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E (2008) Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci 28: 3051–3059. [PMC free article] [PubMed] [Google Scholar]
- Sato C, Barthélemy NR, Mawuenyega KG, B W, Gordon BA, Jockel-Balsarotti J, Sullivan M, Crisp MJ, Kasten T, Kirmess KM, Kanaan NM, Yarasheski KE, Baker-Nigh A, Benzinger TLS, Miller TM, Karch CM, Randall J. Bateman RJ (2018) Tau kinetics in neurons and the human central nervous system. Neuron 97: 1284–1298. [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8: 595–608. [PMC free article] [PubMed] [Google Scholar]
- Shahripour RB, Harrigan MR, Alexandrov AV (2014) N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and Behavior 4: 108–122. [PMC free article] [PubMed] [Google Scholar]
- Shimpi S, Chauhan B, Shimpi P (2005) Cyclodextrins: application in different routes of drug administration. Acta Pharm 55: 139–156. [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G (1997) Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci 17: 2653–2657. [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Murphy MP (2010) Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201: 96–103. [PubMed] [Google Scholar]
- Smith RAJ, Porteous CM, Gane AM, Murphy MP (2003) Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA 100: 5407–5412. [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F, Schmid AW, Rietsch S, Counts SE, Auwerx J (2018) Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552: 187–193. [PMC free article] [PubMed] [Google Scholar]
- Szeto HH (2006) Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J 8: E521–531. [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M (2002) Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis10: 279–288. [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M (2005) β-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinase pathways. J Neurochem 92: 628–636. [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M (2008) Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the β-amyloid precursor protein. J Neurochem 104: 683–695. [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Zhou W, Fung V, Christensen MA, Qing H, Sun X, Song W (2005) Oxidative stress potentiates BACE1 gene expression and Aβ generation. J Neural Transm 112: 455–469. [PubMed] [Google Scholar]
- Velliquette RA, O’Connor T, Vassar R (2005) Energy inhibition elevates β-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer’s disease pathogenesis. J Neurosci 25:10874–10883. [PMC free article] [PubMed] [Google Scholar]
- Virgili J, Lebbadi M, Tremblay C, St-Amour I, Pierrisnard C 1, Faucher-Genest A, Emond V, Julien C, Calon F (2018) Characterization of a 3xTg-AD mouse model of Alzheimer’s disease with the senescence accelerated mouse prone 8 (SAMP8) background. Synapse 72. doi: 10.1002/syn.22025. [PubMed] [CrossRef] [Google Scholar]
- Wagner EM, Jen K-LC, Artiss JD, Remaley AT (2008) Dietary alpha-cyclodextrin lowers LDLC and alters plasma fatty acid profile in LDLr-KO mice on a high-fat diet. Metabolism 57: 1046–1051. [PMC free article] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72: 1858–1862. [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, Landau SM, Rabinovici GD, Jagust WJ (2013) Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 70: 1512–1519. [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wang KK (2015) Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci 38:364–74. [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106: 14670–14675. [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, Ke ZJ (2011) Thiamine deficiency increases β-secretase activity and accumulation of β-amyloid peptides. Neurobiol Aging 32: 42–53. [PubMed] [Google Scholar]
- Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690. [PubMed] [Google Scholar]