- Journal List
- Mol Metab
- v.29; 2019 Nov
- PMC6745493

Dietary sulfur amino acid restriction upregulates DICER to confer beneficial effects
Beatriz A. Guerra
1Department of Biophysics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
2Program in Molecular Biology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
3Department of Biochemistry and Tissue Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
Bruna B. Brandão
1Department of Biophysics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
2Program in Molecular Biology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Silas S. Pinto
1Department of Biophysics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
2Program in Molecular Biology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
3Department of Biochemistry and Tissue Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
4Program in Genetics and Molecular Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
Willian G. Salgueiro
3Department of Biochemistry and Tissue Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
4Program in Genetics and Molecular Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
Evandro A. De-Souza
1Department of Biophysics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
2Program in Molecular Biology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
5Institute of Medical Biochemistry Leopoldo de Meis, Universidade Federal do Rio de Janeiro, Brazil
Felipe C.G. Reis
1Department of Biophysics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Thiago M. Batista
6Department of Structure and Functional Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
Vanessa Cavalcante-Silva
7Department of Psychobiology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Vânia D'Almeida
7Department of Psychobiology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Beatriz A. Castilho
8Department of Microbiology, Immunology and Parasitology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
Everardo M. Carneiro
6Department of Structure and Functional Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
Adam Antebi
9Max-Planck Institute for Biology of Ageing, Cologne, and CECAD, University of Cologne, Cologne, Germany
William T. Festuccia
10Department of Physiology, Instituto de Ciências Biomédicas, Universidade de São Paulo, Brazil
Marcelo A. Mori
1Department of Biophysics, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
2Program in Molecular Biology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil
3Department of Biochemistry and Tissue Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
4Program in Genetics and Molecular Biology, Instituto de Biologia, Universidade Estadual de Campinas, Brazil
Associated Data
Abstract
Objective
Dietary restriction (DR) improves health and prolongs lifespan in part by upregulating type III endoribonuclease DICER in adipose tissue. In this study, we aimed to specifically test which missing dietary component was responsible for DICER upregulation.
Methods
We performed a nutrient screen in mouse preadipocytes and validated the results in vivo using different kinds of dietary interventions in wild type or genetically modified mice and worms, also testing the requirement of DICER on the effects of the diets.
Results
We found that sulfur amino acid restriction (i.e., methionine or cysteine) is sufficient to increase Dicer mRNA expression in preadipocytes. Consistently, while DR increases DICER expression in adipose tissue of mice, this effect is blunted by supplementation of the diet with methionine, cysteine, or casein, but not with a lipid or carbohydrate source. Accordingly, dietary methionine or protein restriction mirrors the effects of DR. These changes are associated with alterations in serum adiponectin. We also found that DICER controls and is controlled by adiponectin. In mice, DICER plays a role in methionine restriction-induced upregulation of Ucp1 in adipose tissue. In C. elegans, DR and a model of methionine restriction also promote DICER expression in the intestine (an analog of the adipose tissue) and prolong lifespan in a DICER-dependent manner.
Conclusions
We propose an evolutionary conserved mechanism in which dietary sulfur amino acid restriction upregulates DICER levels in adipose tissue leading to beneficial health effects.
1. Introduction
Dietary restriction (DR) without malnutrition extends lifespan across species from yeast to primates. It is well established that 25–40% of food restriction with vitamin supplementation can make rodents live longer and protected from metabolic diseases such as type 2 diabetes and obesity, as well as cancer, cardiovascular diseases, and other age-related complications [1], [2], [3]. Evidence that the same occurs in primates (including humans) is mounting, but more studies are required to define how safe and impactful DR is to human beings. For example, calorie restriction may cause undesirable effects such as reduced fertility, osteoporosis, and depression [4]. Dietary interventions seeking to reduce energy intake are thus challenged by low compliance. This has motivated the search for novel treatment regimens that extend life and promote health without necessarily compromising energy intake.
Dietary protein restriction has emerged as a promising alternative to calorie restriction since it is easier to accomplish and has equal beneficial effects in many organisms across the evolutionary spectrum, including humans [5]. It has been demonstrated in flies and mice that the beneficial effects of protein restriction are mediated by a reduction in the levels of dietary essential amino acids, in particular the sulfur amino acids methionine and cysteine [5]. In rodents, dietary methionine restriction and cysteine deprivation (MR) prolongs lifespan and increases stress resistance [6]. Strikingly, MR leads to weight loss and reduces adiposity despite increasing food intake [6]. This is primarily due to an increase in energy expenditure, which is partially explained by brown/beige adipocyte activation [7].
Part of the beneficial effects of DR is thought to be mediated by the adipose tissue [8]. Changes in fat mass or function affect the outcome of DR and impact the onset of metabolic, age-related diseases [8], [9]. Among the pathways affected by DR in adipose tissue, our group is particularly interested in the miRNA processing pathway. Overall expression of adipose tissue miRNAs is down-regulated with aging [10], obesity [11], and lipodystrophy [12] in mammals. In contrast, DR up-regulates a wide spectrum of miRNAs in mouse adipose tissue [10]. This pattern is mediated by changes in the expression of components of the miRNA processing pathway, particularly the type III endoribonuclease DICER, and appears to be restricted to white adipose tissues [10]. Mice lacking Dicer in adipocytes show insulin resistance, “whitening” of the brown adipose tissue, age-associated partial lipodystrophy, and premature mortality rate [13]. In Caenorhabditis elegans, loss-of-function mutations in the Dicer gene (dcr-1) result in shorter lifespan [10]. On the other hand, DR does not improve insulin sensitivity or adipose tissue oxidative function in fat-specific Dicer knockout mice (ADicerKO) [13], suggesting that adipose tissue Dicer is necessary for at least some of the beneficial effects of DR. Importantly, overexpression of Dicer in the worm's closest analog to adipose tissue – the intestine – results in a mild increase in lifespan and robust stress resistance [10].
To explore the molecular mechanisms through which DICER is upregulated by DR, we performed an in vitro nutrient screen and follow-up validation studies in vivo to define that dietary methionine restriction is both sufficient and necessary to explain how DR up-regulates DICER in adipose tissue of mice. We also demonstrate that adipose tissue DICER is required for DR- and MR-induced adiponectin upregulation and recruitment of newly formed beige adipocytes. Finally, we show that DR and MR also upregulate DCR-1 in the intestine of C. elegans and increase lifespan in wild-type worms, but not in worms lacking dcr-1. Thus, we conclude that DR acts via dietary sulfur amino acid restriction to upregulate DICER and confer beneficial health effects in mice and worms.
2. Methods
2.1. Animals and nutritional interventions
2.1.1. Animals
Animals were kept at 22 ± 2 °C under a light/dark cycle of 12 h and ad libitum access to water and food, unless otherwise stated in each experimental condition. Groups of 4–8 males were used throughout this study. Interventions started when the mice were 10–14 weeks old (20–28 g). We used C57Bl/6J mice, adipocyte-specific Dicer knockout mice (Dicerlox/lox::Adiponectin-Cre = ADicerKO) and their respective littermate controls (Dicerlox/lox = Lox) [10], and whole-body Gcn2 (General Control Nonderepressible 2) knockout mice (Gcn2KO) [14] from CEDEME-UNIFESP. Adipocyte-specific Raptor (Regulatory-associated protein of mTOR) knockout mice (Raptorlox/lox::Adiponectin-Cre = ARaptorKO) and their respective control littermates (Raptorlox/lox = Lox) [15], and whole-body adiponectin knockout mice (AdipoqKO) [16] were obtained from the animal facility at the Institute of Biomedical Sciences, Universidade de São Paulo. All mice were backcrossed to C57Bl/6J background. All procedures were conducted in accordance with the National Institute of Health Laboratory's Guide to Care and Uses of Laboratory Animals [17] and approved by the IACUC of the Universidade Federal de São Paulo and Universidade Estadual de Campinas (CEP-180/12, CEP-0218/11, CEP-0237/12, CEUA-4603261015 and CEUA-4791-1/2018).
2.1.2. Nutritional interventions
For dietary restriction, we used a protocol previously validated by the National Institute on Aging [18] and published elsewhere by our group [10]. In brief, mice were fed with the NIH31/NIA-Fortified chow and kept in DR (10% during the first week, 25% during the second, and 40% from the third on) for 4 weeks unless stated otherwise. Using this protocol, we found significant increases in miRNA processing in adipose tissue after 12 weeks [10] and up-regulation of DICER after just one week [13].
For diet supplementation, NIH31/NIA-Fortified was supplemented with either one of the following nutrients: 1) 0.14% l-methionine, 2) 0.21% l-cysteine, 3) 7.50% casein, 4) 12.50% sucrose, 5) 0.14% vitamin C (all from Sigma–Aldrich®), or 6) 1.74% soybean oil (SOYA®). The NIH31/NIA-Fortified diet has a macronutrient ratio of 17.90% protein, 46.60% carbohydrate and 4.70% fat, and a caloric value of 3.0 kcal/g. The supplementations were meant to increase specific nutrient intake to match the ad libitum intake without significantly changing (<10%) the caloric value of the diet or the balance of other macronutrients. This was possible for all interventions except for sucrose supplementation, in which the macronutrient ratio was: 52.50% carbohydrate (12.70% more than in the NIH31/NIA-Fortified diet), 15.90% protein and 4.20% lipid with 3.1 kcal/g of caloric value. To further increase the proportion of carbohydrates without dramatically altering the proportion of other macronutrients, we offered the mice sugared water beginning in the second week of the diet. Considering the average daily water intake and the amount of sugar needed to recover the carbohydrate intake of the ad libitum group, a 20 mg/mL sucrose solution was offered during the second week (25% food restriction) and a 47 mg/mL sucrose solution from the third week on (during the 40% food restriction period). Calorie intake of the animals fed sucrose-supplemented diet increased by up to 15%, which was still lower than the calorie intake of the ad libitum group. Supplementations were performed in-house by mixing nutrients with powdered diet. After homogenizing the diets, we pelleted the chow and let it dry at 60 °C for approximately 12 h.
For methionine restriction, animals were subjected to 86% dietary methionine restriction with cysteine deprivation (MR) for 12 weeks as described elsewhere with slight modifications [19]. In some cases, MR diet was supplemented with cystine (MRC) to match the levels of cysteine in the control group. The diets were produced according to [19] and manufactured by Prag Soluções®. For high fat diet, animals were subjected to diet containing 60% calories from fat during 8 weeks as previously described in Belchior and coauthors (2015) [20]. For protein restriction, mice were subjected to 50% dietary protein restriction for 14 weeks according to a previous publication [21].
2.2. Cells and in vitro nutrient restriction
3T3-F442A preadipocytes were cultured in DMEM with 10% fetal bovine serum (Sigma–Aldrich®), 2 mM l-glutamine (Sigma–Aldrich®), 100 U/mL penicillin (Life Technologies®), and 100 μg/mL streptomycin (Life Technologies®). Cells were maintained in culture dishes in an incubator at 37 °C with 5% CO2. After reaching confluency of approximately 70%, they were rinsed with sterile PBS and control medium or nutrient restriction medium was added for 48 h. For nutrient restriction, we used culture media modified from DMEM Base D5030 or D0422 (Sigma–Aldrich®) supplemented with 2% fetal bovine serum. Restriction media had 40% less of the specific nutrient.
2.3. Biochemical and metabolic analyzes
2.3.1. Glucose tolerance test (GTT)
Mice were subjected to 12 h fasting and injected intraperitoneally with d-glucose (1 g glucose/kg body weight). Blood glucose levels were measured using a glucometer (Roche®) before and after glucose administration. The experiment was performed 3 days prior to euthanasia [22].
2.3.2. Sample collection
Animals were sacrificed in the period between 11:00 AM and 2:00 PM, and blood was immediately collected in microtubes, stored at room temperature for approximately 15 min, and then placed at 4 °C. After obtaining all blood samples, they were centrifuged at room temperature for 15 min at 344×g and the serum was collected and stored at −80 °C for further analysis. Body composition was measured by weighing the tissues shortly after euthanasia. These tissues were transferred to microtubes and immediately frozen in dry ice and stored at −80 °C for further analysis.
2.3.3. Serum parameters
Serum insulin, adiponectin, and leptin was measured using Millipore® (insulin) and R&D Systems® (adiponectin and leptin) ELISA kits following manufacturer's protocols. Serum triglycerides level was quantified using the LabTest® colorimetric kit following the manufacturer's instructions. Serum homocysteine, cysteine, and glutathione levels were measured at the Laboratory of Inborn Errors of Metabolism of the Universidade Federal de São Paulo by high-performance liquid chromatography with isocratic elution and fluorometric detection [23].
2.3.4. DNA damage
DNA damage was measured in subcutaneous inguinal adipose tissue by assessing 8-oxo-2′-deoxyguanosine (8OHDG) levels. Adipose tissue was homogenized in 0.1 M phosphate buffer, pH 7.4 with 1 mM EDTA using the Bullet Blender® apparatus. The homogenate was centrifuged at 1,000×g, 10 min, 4 °C and the supernatant was subjected to DNA extraction using the DNeasy® Kit from Qiagen® following the manufacturer's instructions. 2 μg DNA was then digested at 30 °C for 30 min using 2 U Mung Bean nuclease (Pharmacia®) in NEBuffer 1 (NE Biolabs®) to eliminate single strands of DNA and RNA. We then added 5 U of Antarctic Phosphatase and its buffer (NE Biolabs®) and incubated the reaction for 15 min at 37 °C. After the incubation, DNA was purified and precipitated using phenol:chloroform followed by sodium acetate:ethanol. The pellet was resuspended in sterile water, DNA concentration was determined using NanoDrop 2000c spectrophotometer (Thermo Scientific®), and 630 ng of DNA was used in the 8OHDG assay kit from Cayman Chemical® following the manufacturer's protocol.
2.3.5. RT-qPCR
Quantitative analysis of mRNAs was performed according to our previously described protocols [13]. Primer sequences are available upon request. We used 36B4 for mRNA normalization.
2.3.6. Western blotting (SDS-PAGE)
For western blotting, we used the protocol previously described in Mori et al., 2008 [22]. The antibodies were: anti-Dicer (13502, Abcam®), anti-Ago2 (2897, Cell Signaling®), anti-beta-tubulin (2146, Cell Signaling®), anti-rabbit IgG-HRP (RPN4301, GE®). Scion Image® software was used for the quantification of bands by densitometry. We used beta-tubulin or Ponceau S dye (Sigma–Aldrich®) as loading controls.
2.4. C. elegans assays
Worms were kept in standard culture conditions [25]. Worms were grown in OP50-1 unless otherwise indicated. The following strains were used in this study: wild-type N2 Bristol, PD8753 [dcr-1(ok247) III/hT2 [bli-4(e937) let-?(q782) qIs48] (I; III)], DA1116 [eat-2(ad1116)], MAM89 [mamIs89: pdcr-1::GFP + myo-3::RFP], MAM129 [eat-2 (ad1116) II; mamIs89], MAM13.2 [mamIs13: pdcr-1::dcr-1::GFP + pUN24 (pY66H1B.3::Y66H1B.3::GFP) + pJK590 (plag-2::GFP)], and MAM35 [eat-2 (ad1116) II; mamIs13]. Constructs harboring the dcr-1 promoter and the dcr-1 coding sequence fused with GFP were generated as previously described [10], [26] and injected in the germline. myo-3::RFP and pUN24/pJK590 were used as co-injection markers due to their selective localization in the pharynx and germline, respectively, with no obvious intestinal expression. Transgene integration was performed using UVC light and worms were outcrossed for at least 4 times with N2 prior to experiments.
2.4.1. Lifespan assays
Lifespan and RNAi assays were performed as described in Ferraz et al., 2016 [27] with slight modifications. Briefly, for each replicate, approximately 120 synchronized day 0-adult worms were transferred to Nematode Growth Medium (NGM) plates containing OP50-1 or HT115 (DE) bacteria. Lifespan assays were performed at 20 °C in plates containing 50 μg/mL FUdR unless stated otherwise. Beginning at day zero of adulthood, dead worms were scored, and bag-of-worms or escapers were censored until the death of the last worm. The eat-2(ad1116) mutant was used as a genetic model of dietary restriction as previously described [28]. The protocol of sDR was performed as in Greer and Brunet, 2009 [29]. In these experiments, worms were maintained in ad libitum plates on OP50-1 until day 4 of adulthood when they were transferred to ad libitum or sDR plates. Worms were transferred to new plates every 2 days and no FUdR was used. Bacteria concentration was 5 × 1011 or 5 × 108 in ad libitum or sDR plates, respectively, and 100 μg/mL ampicillin was used as bacteriostatic.
For RNAi, synchronized eggs were transferred to NGM plates containing HT115 (DE3) harboring the empty vector (L4440) or dcr-1 RNAi and 1 mM of IPTG. Worms were maintained in these RNAi plates until day 1 of adulthood and then let to lay eggs for 1 h in new RNAi plates. The hatched larvae developed until adulthood and were then transferred to new RNAi plates containing 50 μg/mL FUdR and lifespan was monitored. Metformin was mixed to NGM at the concentration of 50 mM according to [30]. Worms were transferred to metformin or vehicle (water) plates when they reached adulthood.
2.4.2. C. elegans dcr-1 expression
In order to measure transgene fluorescence and assess dcr-1 promoter activity (mamIs89) and protein abundance (mamIs13) in intestinal cells, adult worms (day 0) were transferred to plates supplemented with FUdR, and left to grow at 20 °C under standard culture conditions until image acquisition. Two percent agarose pad slides were mounted, and drops of 25 mM levamisole were used in order to immobilize the animals. Samples were examined using a Zeiss Axio Observer. Z1 inverted microscope (Carl Zeiss AG, Germany) over a 4× objective (40× magnification). Fifteen to thirty worms were used to measure GFP intensity in the distal intestine by calculating CTCF = integrated density - (area of selected cell X mean fluorescence of the background).
RT-qPCR was conducted in approximately 150 synchronized day 0 worms in order to measure the expression of dcr-1::GFP mRNA. Experiments were performed as described in 2.3.5. and normalized to his-10 expression.
2.5. Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM) and analyzed in the software GraphPad Prism®. We used Student t-test for two group comparisons, and ANOVA for more than two groups. When data had more than two categorical independent variables, we used two-way ANOVA with Tukey's post-test. Log-rank test was performed for longevity assays in C. elegans. We adopted p < 0.05 as a limit of significance.
3. Results
3.1. DICER is upregulated in adipose tissue by dietary sulfur amino acid restriction
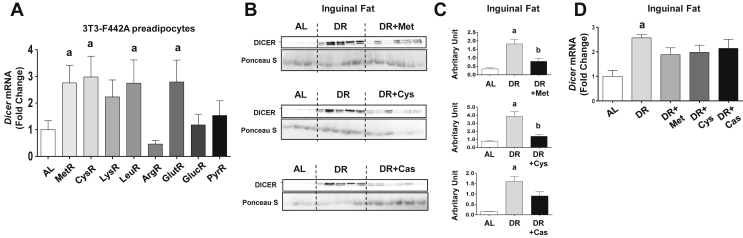
In addition to decreasing calorie intake, DR reduces the availability of essential nutrients in the diet and, consequently, in the blood stream. To test whether it is the reduction of essential nutrient availability which upregulates DICER in adipocytes, we performed a nutrient screen in 3T3-F442A preadipocytes to determine which nutrients, when restricted by 40%, result in Dicer upregulation. Among the conditions that we tested, we found consistent increases in Dicer mRNA expression in cells exposed to methionine or cysteine restricted medium (Figure 1A). Restriction of other amino acids also upregulated Dicer mRNA, independently of whether they were essential or not, whereas glucose or pyruvate restriction had no effect (Figure 1A).
DICER expression in preadipocytes and subcutaneous inguinal adipose tissue is controlled by sulfur amino acid levels. A = Expression of Dicer mRNA in 3T3-F442A preadipocytes incubated for 48 h with 40% methionine (MetR, N = 10), cysteine (CysR, N = 7), lysine (LysR, N = 9), leucine (LeuR, N = 8), arginine (ArgR, N = 6), glutamine (GlutR, N = 9), glucose (GlucR, N = 14), or pyruvate (PyrR, N = 6) restricted medium or complete medium (N = 14). At least two independent experiments were performed. B–D = Mice were subjected to 4 weeks of ad libitum diet (AL, N = 3), dietary restriction (DR, N = 5) or DR plus 40% of methionine (DR + Met, N = 6), cysteine (DR + Cys, N = 6) or casein (DR + Cas, N = 6). B = protein abundance in inguinal adipose tissue as measured by western blotting. C = band densitometry. Membrane staining with Ponceau S Red was used as loading control. D = Dicer mRNA expression. Results are presented as mean ± SEM. p < 0.05: a, compared to the AL group; b, compared to the DR group. One-way ANOVA test with Tukey's post-test.
To test whether amino acid restriction was necessary for the effects of DR in vivo, we supplemented the DR regimen with 40% more of methionine, cysteine, or casein to match the amino acid levels ingested by the ad libitum group. Importantly, the supplemented diets were practically isocaloric (approximately 3.0 kcal/g each) when compared to the DR group. We observed that the addition of sulfur amino acids, as well as of casein, partially or almost completely reversed the effect of DR on DICER protein abundance (Figure 1B,C). This was also observed at the mRNA level, although less prominently (Figure 1D). Argonaute 2 (AGO2) – an RNase required for miRNA function downstream of DICER [31] – was similarly affected by DR, but sulfur amino acid supplementation did not reverse this effect (Figure S1A,B).
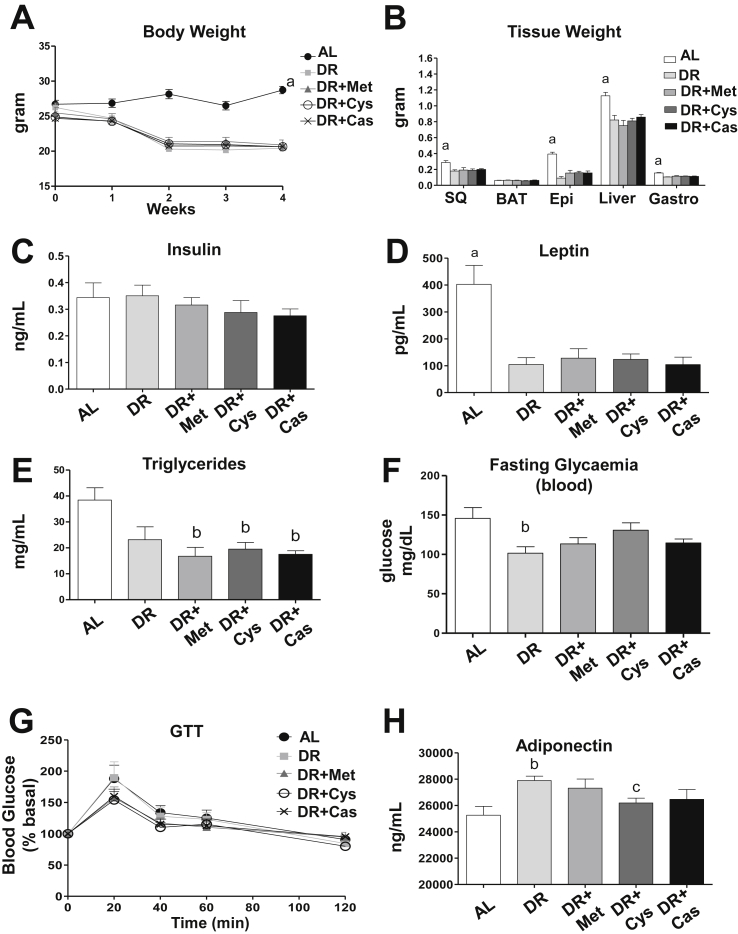
Consistent with a specific role for dietary amino acids in DICER regulation, supplementation of DR with carbohydrate (i.e. sucrose) or fat (i.e. vegetable oil) did not interfere with the effect of DR over DICER protein or mRNA levels (Figure S2). Oil supplementation did not significantly affect calorie availability or nutrient balance of the DR diet, while sugar supplementation resulted in a small reduction in protein and lipid intake (11%) and a mild increase in calorie intake (about 15%). Mice fed the oil-supplemented diet lost weight in the same way as the DR group but kept their fasting glycaemia at similar levels in relation to the ad libitum group (Figure S3). On the other hand, mice fed the sugar-supplemented diet maintained their body weight and fasting glycaemia like the ad libitum group (Figure S3). In contrast, supplementation with methionine, cysteine, or casein did not interfere with how DR affected body weight (Figure 2A), tissue mass (Figure 2B) or serum levels of insulin (Figure 2C), leptin (Figure 2D) and triglycerides (Figure 2E). These findings suggest that although DICER abundance in adipose tissue is specifically regulated by amino acids, this regulation does not appear to overcome the DR effects over adiposity. However, the ability of DR to reduce fasting glucose was mildly attenuated by sulfur amino acid supplementation (Figure 2F), whereas no significant changes were found in a glucose tolerance test (Figure 2G). In addition, serum level of adiponectin - an important antidiabetic and cardioprotective adipokine [32] - which is increased by DR, was partially reduced by amino acid supplementation, particularly cysteine (Figure 2H), consistent with changes in DICER abundance in adipose tissue.
Dietary sulfur amino acid restriction contributes to the metabolic effects of DR. Mice were subjected to 4 weeks of ad libitum diet, dietary restriction (DR), or DR plus methionine (DR + Met), cysteine (DR + Cys), or casein (DR + Cas) supplementation (N = 6 per group). A = Body weight. B = Tissue weight at sacrifice. SQ (subcutaneous inguinal adipose tissue), BAT (brown adipose tissue), EPI (epididymal adipose tissue), Liver and Gastro (gastrocnemius muscle). C = Serum insulin. D = Serum leptin. E = Serum triglycerides. F = Blood glucose levels in animals fasted for 12 h (N = 6 per group). G = glucose tolerance test performed after 12 h fasting. H = serum adiponectin. Results are presented as mean ± SEM. p < 0.05: a, compared to others groups; b, compared to the ad libitum (AL) group; c, compared to the DR group. One-way ANOVA test with Tukey's post-test.
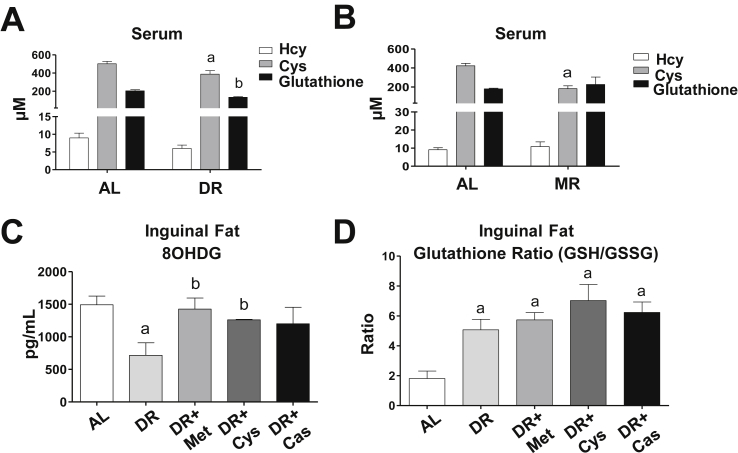
Another important beneficial effect of DR is protection from oxidative stress via activation of an endogenous antioxidant response [33]. Sulfur amino acids play a role in antioxidant defenses, in part because they are necessary for the synthesis of glutathione, a major intracellular antioxidant [34]. Indeed, we observed that animals subjected to DR or MR had lower levels of cysteine and glutathione in the blood, with no significant changes in homocysteine (Figure 3A,B). To determine whether DR and amino acid supplementation would alter the redox status of the adipose tissue, we measured the levels of 8-oxo-2′-deoxyguanosine (8OHDG) – a marker of DNA oxidation - and the ratio between reduced (GSH) and oxidized (GSSG) glutathione in this tissue. There were lower levels of 8OHDG upon DR, and this was reversed by supplementation with sulfur amino acids (Figure 3C). On the other hand, despite the lower levels of blood glutathione, the glutathione turnover ratio (GSH/GSSG) was increased by DR in adipose tissue and not affected by amino acid supplementation (Figure 3D). These data indicate that DR promotes an antioxidant protection that is mediated by sulfur amino acids, but this seems to be independent of the antioxidant capacity of glutathione.
Dietary sulfur amino acid restriction promotes an antioxidant response. (A, C, D) Mice were subjected to 4 weeks of ad libitum diet (AL, N = 8), dietary restriction (DR, N = 6) or DR plus 40% of methionine (DR + Met, N = 6), cysteine (DR + Cys, N = 6), or casein (DR + Cas, N = 6). B = Mice were subjected to dietary methionine restriction diet (MR, N = 4) or ad libitum diet (AL, N = 5) for 12 weeks. A and B = Serum homocysteine, cysteine, and glutathione levels as measured by HPLC. C = 8-Oxo-2′-deoxyguanosine (8OHDG) levels in subcutaneous inguinal adipose tissue (N = 5 per group). D = Reduced and oxidized glutathione ratio (GSH/GSSG) in subcutaneous inguinal adipose tissue (N = 6 per group). Results are presented as mean ± SEM. p < 0.05: a, compared to the ad libitum (AL); b, compared to the DR group. One-way ANOVA test with Tukey's post-test.
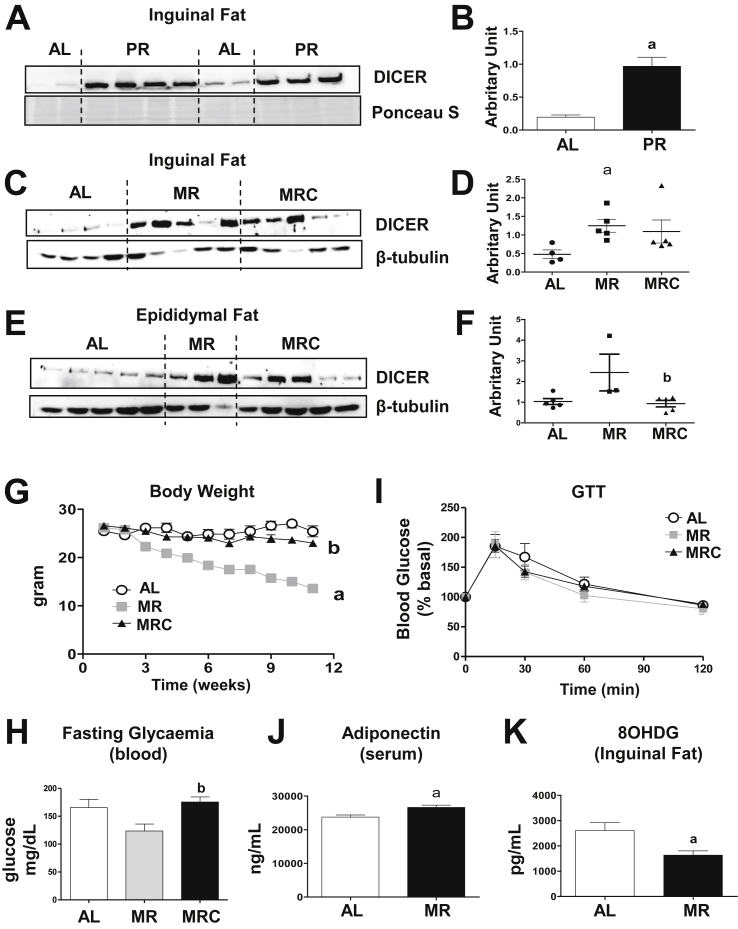
Next, to test whether dietary sulfur amino acid restriction in vivo is sufficient to mimic DR, we measured DICER abundance in inguinal adipose tissue samples of mice subjected to isocaloric 50% protein restriction diet (PR) or 86% methionine restriction, cysteine deprivation diet (MR). To assess the role of de novo cysteine synthesis by methionine, we had a group of animals in which the MR diet was supplemented with cystine (MRC). In both paradigms, similar to in vitro conditions, PR and MR increased DICER abundance in inguinal fat (Figure 4A–D), while cystine supplementation in the MR diet tended to reverse this pattern except for one outlier (Figure 4C,D). MR also led to a similar induction of DICER in epididymal fat (Figure 4E,F), a reduction in body weight (Figure 4G) and a trend toward reduction in fasting blood glucose levels (Figure 4H), while cystine supplementation blunted these effects. However, when normalized by basal glucose levels, glucose tolerance was not different between the groups (Figure 4I). Furthermore, MR increased serum adiponectin (Figure 4J) and reduced adipose tissue 8OHDG levels (Figure 4K), reinforcing the association between these parameters and DICER expression. Thus, our data demonstrate that reduced availability of methionine in dietary restriction protocols leads to effects that include DICER upregulation, reduced oxidative stress in adipose tissue, and increased blood adiponectin levels.
Dietary sulfur amino acid restriction mimics DR. (A, B) Mice were subjected to dietary protein restriction (PR, N = 7) or ad libitum diet (AL, N = 4) for 14 weeks. (C–H) Mice were subjected to methionine restriction (MR, N = 5), methionine restriction plus cystine supplementation (MRC, N = 5), or ad libitum diet (AL, N = 4) for 12 weeks. A, C. and E = DICER abundance in adipose tissue as measured by western blotting. B, D and F = band densitometry. Protein abundance was normalized by Ponceau S or beta-tubulin. G = Body weight (AL and MRC, N = 5 and MR, N = 6). H = Blood glucose levels in animals fasted for 12 h (N = 5 per group). I = Glucose tolerance test performed after 12 h fasting (N = 5 per group). J = Serum adiponectin levels (AL, N = 5 and MR, N = 4). K = 8-Oxo-2′-deoxyguanosine (8OHDG) levels in subcutaneous inguinal adipose tissue (N = 4 per group). Results are presented as mean ± SEM. p < 0.05: a, compared to the ad libitum (AL) group; b, compared to the MR group. In B, J and K, unpaired Student t test. In D, F, G, H, and I, one-way ANOVA test with Tukey's post-test.
3.2. Mechanisms that control DICER levels in adipose tissue
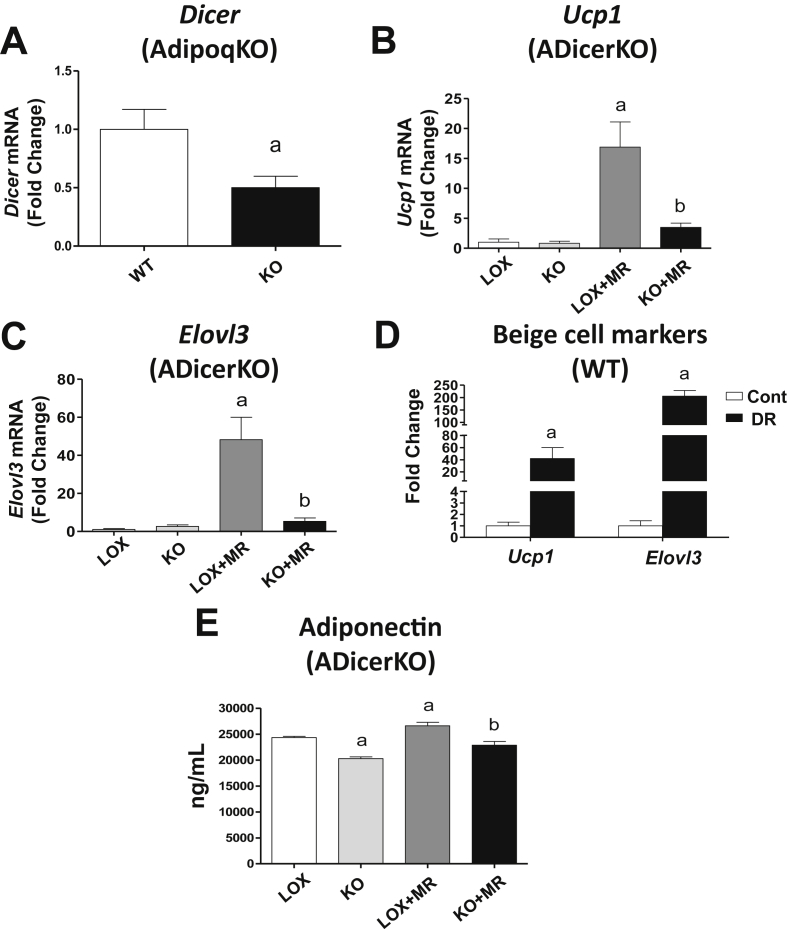
To understand how MR upregulates DICER, we used mice lacking the nutrient sensors (i) GCN2 (general control nonderepressible 2) - an eIF2α kinase which is activated by uncharged tRNAs under conditions of amino acid restriction [35] – or (ii) Raptor – a necessary component of the mechanistic target of rapamycin complex 1 (mTORC1) which is activated by amino acids and growth hormones [36]. Inguinal adipose tissue DICER levels were not affected by either genotype (Figure S4A–D). DR and MR have been proposed to induce longevity through mitohormesis – a phenomenon in which mild increases in prooxidant species may result in beneficial effects through activation of an endogenous antioxidant pathway and reduce overall oxidative damage [37], [38]. However, DR does not seem to act via prooxidant species to upregulate adipose tissue DICER, since vitamin C supplementation (VitC) did not interfere with the DR effect (Figure S4E,F). Finally, as DR and MR upregulate adiponectin (Figure 2, Figure 4J), we tested whether adiponectin is a positive regulator of Dicer expression. Consistent with this hypothesis, adiponectin knockout mice exhibited lower levels of Dicer mRNA in subcutaneous inguinal adipose tissue (Figure 5A).
Dicer is required for the effects of MR on subcutaneous adipose tissue. A = Dicer mRNA was measured in subcutaneous inguinal adipose tissue of adiponectin knockout mice (AdipoqKO) or C57BL/6J wild type (WT) mice (N = 6 per group). (B, C, E) Fat-specific Dicer knockout mice (ADicerKO) or floxed controls (LOX) were subjected to ad libitum diet or methionine restriction (MR) for 12 weeks (LOX, N = 5; KO N = 5; LOX + MR N = 7; KO + MR group N = 4). B = Ucp1 and C = Elovl3 mRNA expression in subcutaneous inguinal adipose tissue. (D) C57BL/6J wild type mice were subjected to dietary restriction (DR) or ad libitum (Cont) for 4 weeks (N = 6 per group). D = Ucp1 and Elovl3 mRNA expression in subcutaneous inguinal adipose tissue. E = Serum adiponectin levels in ADicerKO mice and LOX controls. Results are presented as mean ± SEM. In A, a = p < 0.05 compared to WT (Student t-test). In B, C and E, a = p < 0.05 compared to LOX and b = p < 0.05 compared to LOX + MR (Two-way ANOVA with Tukey's post-test). In D, a = p < 0.05 compared to Cont (Student t-test).
3.3. DICER is required for the browning effects of MR in mice
Restriction of dietary sulfur amino acids promotes thermogenesis in white adipose tissue via recruitment of beige adipocytes and upregulation of UCP1 [39]. Here we tested if this phenomenon is dependent on DICER. Indeed, MR led to upregulation of beige adipocyte markers (i.e. Ucp1 and Elovl3) in subcutaneous inguinal adipose tissue of wild type mice (Lox), and this was strongly suppressed in adipocyte-specific Dicer knockout mice (ADicerKO) (Figure 5B,C). DR resulted in marked upregulation of the same markers in inguinal adipose tissue of wild type mice (Figure 5D). Adiponectin induces Ucp1 expression in adipose tissue [40]. Consistent with a model in which adiponectin and DICER co-regulate each other, serum adiponectin levels are reduced in ADicerKO mice (Figure 5E).
3.4. DCR-1 is required for the longevity effects of MR and DR in C. elegans
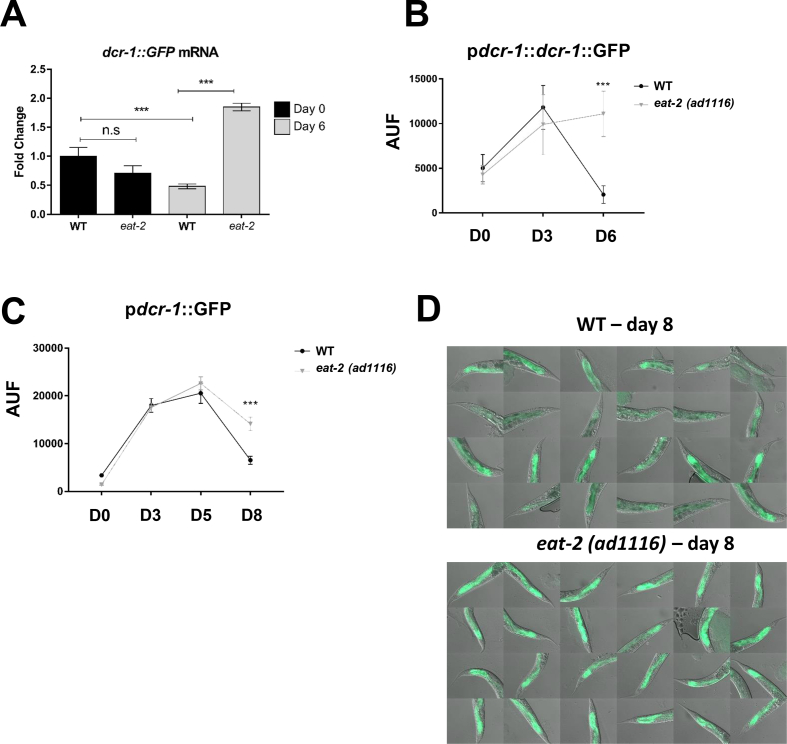
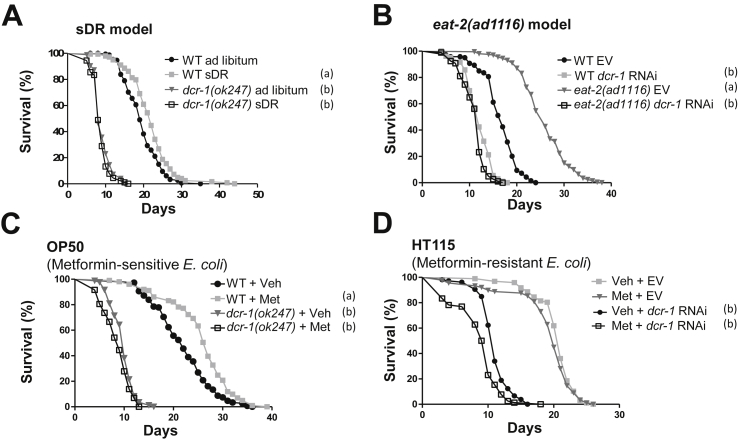
Finally, we took advantage of C. elegans short lifespan and conserved miRNA pathway to study if DR and MR require the DICER ortholog gene (dcr-1) to promote longevity. The eat-2(ad1116) mutation - a genetic model of DR which leads to decreased pharyngeal pumping and reduces food intake of the worm - inhibited the downregulation of DCR-1 that occurs in the worm's intestinal cells (the analog of mammalian adipose tissue) with aging (Figure 6A–D). This mechanism is controlled at least in part at the transcriptional level, given that the dcr-1 promoter is by itself downregulated with aging in ad libitum worms but less so in DR worms (Figure 6C,D). Importantly, DCR-1 was required for longevity induced by two different protocols of DR in C. elegans, namely bacterial dilution in semi-solid medium (sDR) (Figure 7A) or the eat-2(ad1116) mutation (Figure 7B) (Table S1). Since worms feed on bacteria, methionine restriction requires manipulation of the live food source. This can be achieved by treating OP50 E. coli with metformin [30]. On the other hand, HT115(DE3) E. coli are resistant to metformin and do not serve as a model of MR [30]. Indeed, metformin increased lifespan of wild type worms on OP50 but not on HT115 (Figure 7C) (Table S1). Consistent with a role of DCR-1 in MR-mediated lifespan extension, metformin did not promote longevity in dcr-1 null mutants on OP50 (Figure 7C) (Table S1) and decreased lifespan in worms exposed to dcr-1 RNAi on HT115 (Figure 7D). Moreover, dcr-1 reporter worms grown on OP50 plates and exposed to metformin exhibited upregulation of dcr-1 promoter activity and DCR-1 abundance in intestinal cells (Figure S5). Hence, DR and MR upregulate DCR-1 levels in intestinal cells of C. elegans and increase longevity in the worm via a DCR-1-mediated pathway.
Age-related decrease in DCR-1 expression is mitigated in eat-2(ad1116) mutants - a dietary restriction (DR) model in C. elegans. A = Worms expressing the dcr-1 reporter transgene mamIs1 (pdcr-1::dcr-1::gfp) in the wild type (WT) or eat-2(ad1116) backgrounds had their RNA extracted at the day 0 and 6 of adulthood and dcr-1::gfp mRNA was measured using RT-qPCR. N = 3 pools per group with at least 50 worms in each pool. ***p < 0.001 (One-way ANOVA with Tukey's post-test). B = GFP fluorescence was assessed in the posterior intestine at day 0, 3 and 6 of adulthood. The experiment was performed twice with at least 8 worms per condition. C = dcr-1 promoter activity was measured in the posterior intestine of worms expressing the mamIs3 (pdcr-1::gfp) transgene on WT or eat-2(ad1116) backgrounds. GFP fluorescence was measured using 15–20 worms per day and condition and experiments were repeated three times. ***p < 0.001, between wild-type and eat-2(ad1116) within the same timepoint (Student t-test). D = Representative images of pdcr-1::gfp expressing worms at the 8th day of adulthood. AUF = Arbitrary Units of Fluorescence.
Dietary and methionine restriction require DCR-1 to prolong lifespan in C. elegans. A = Wild type (WT) and dcr-1(ok247) mutant worms in semi-solid DR (sDR) or ad libitum conditions [N = 177, 164, 90 and 95 worms in the WT ad libitum, WT sDR, dcr-1(ok247) ad libitum, and dcr-1(ok247) sDR, respectively]. Sum of 2 independent replicates. The concentration of OP50 bacteria was 5 × 1011 and 5 × 108 bacteria/mL in the ad libitum and sDR conditions, respectively. B = eat-2(ad1116) and WT worms on dcr-1 RNAi- or empty vector (EV)-containing HT115 bacteria (N = 139, 203, 201 and 185 worms in the WT EV, WT dcr-1 RNAi, eat-2 EV, and eat-2 dcr-1 RNAi, respectively). Sum of 2 independent replicates. C = Lifespan of WT and dcr-1(ok247) worms in response to metformin (Met) or vehicle (Veh) in metformin-sensitive OP50 bacteria (N = 149, 101, 84 and 72 worms in the WT + Veh, WT + Met, dcr-1(ok247)+Veh, and dcr-1(ok247)+Met, respectively). Representative experiment of 3 replicates. D = Lifespan of WT worms on EV or dcr-1 RNAi in response to Met or Veh in HT115 metformin-resistant HT115 bacteria (N = 97, 89, 106 and 78 worms in the Veh + EV, Met + EV, Veh + dcr-1 RNAi and Met + dcr-1 RNAi, respectively). Representative experiment of 3 replicates. Data were compared using the log-rank test. P < 0.05: a, compared to WT ad libitum (A), WT EV (B) or WT + Veh (C); b, compared to respective WT (A and C) or EV (B and D). Full data and statistics are shown in Table S1.
4. Discussion
DICER is a key enzyme required for the expression and function of several small RNA species, among them miRNAs [41]. Over the past few years, our group and others have demonstrated that DICER abundance dynamically changes in adipose tissue in response to metabolic stimuli of different sorts. For example, DICER is downregulated in fat of lipodystrophic patients [12] and in animal models of aging [10], progeria [42], and obesity [42]. These changes in DICER levels reflect the pool of miRNAs in adipocytes [10] and, importantly, markedly affect the levels of miRNAs in circulation [43]. Consistent with the conditions where DICER appear downregulated, adipocyte-specific Dicer knockout (ADicerKO) mice exhibit “whitening” of the brown adipose tissue [12] and a higher risk of premature mortality [12]. On the other hand, interventions that are metabolically beneficial and lead to increased lifespan, such as DR, upregulate DICER in adipose tissue [10]. Interestingly, this upregulation occurs rapidly (within half a week of a 10% DR protocol), anticipates the metabolic effects of DR, and is sustained for as long as DR is maintained [13]. This led us to hypothesize that these dynamic changes in adipose tissue DICER abundance are required for the beneficial effects of DR, in part by ensuring the capacity of adipocytes to express miRNAs under conditions of metabolic stress. Indeed, ADicerKO mice are unable to increase mitochondrial content in fat and improve insulin sensitivity at the whole-body level in response to DR [13]. Similarly, our studies in C. elegans demonstrate that upregulation of dcr-1 in the intestine of worms is sufficient to improve oxidative stress response [10] and necessary for longevity induced by DR and MR (this study).
While the benefits of maintaining increased DICER levels in adipose tissue have been described, the mechanisms through which DR elicits this upregulation remained unknown. We hypothesized that nutrient restriction could affect DICER levels. Indeed, we found that adipose tissue DICER is sensitive to the levels of sulfur amino acids. Under DR, PR, or MR, DICER levels are upregulated. On the other hand, when methionine or cysteine are supplemented to DR, DICER abundance is partially reversed toward the levels of ad libitum fed mice, suggesting that dietary sulfur amino acid restriction is both necessary and sufficient to upregulate DICER in adipose tissue. Importantly, sulfur amino acid restriction prolongs lifespan [6], [34], [44], improves insulin sensitivity [45], [46], promotes adipose tissue browning [39], protects against oxidative stress [47], [48], among other features that have been also directly or indirectly associated with adipose tissue DICER upregulation.
Our data also demonstrate the close relationship between the levels of sulfur amino acids in the diet and various parameters, some of which also relate to DICER levels, like the levels of adiponectin in the blood and 8OHDG in adipose tissue. Importantly, we observed that adiponectin controls Dicer expression in adipose tissue. On the other hand, the absence of Dicer in adipocytes leads to downregulation of blood adiponectin. Together, these results indicate that adiponectin and DICER co-regulate each other in adipocytes, and this mechanism is promoted by sulfur amino acid restriction.
Several studies (including this one) have shown upregulation of adiponectin in response to MR ([49] and Figure 4I). These effects are usually accompanied by upregulation of Ucp1 in inguinal adipose tissue [39] – a bona fide marker of beige adipocyte recruitment [50]. Adiponectin promotes browning by directly binding to anti-inflammatory M2 macrophages, inducing their proliferation and consequent stimulation of beige adipocytes [51]. This suggests that browning induced by MR may involve at least in part M2 macrophages. MR-induced browning also requires the presence of DICER in adipocytes, which, in turn, is a necessary condition for proper adiponectin production. We thus propose a model in which adiponectin leads to DICER upregulation, favoring more adiponectin production and promoting induction of browning. Hence, in the absence of adiponectin or DICER, browning is not sustained.
DR and MR also upregulate DCR-1 in C. elegans intestine, and they require DCR-1 to prolong lifespan in nematodes, revealing hundreds of millions of years of evolutionary conservation. C. elegans does not have an obvious adiponectin ortholog but has three orthologs of the adiponectin receptors that play a role in lipid metabolism, stress response, and aging [52], [53]. It is yet to be investigated whether they participate in the effects of sulfur amino acid restriction or if they control DCR-1 expression in C. elegans. More importantly, this degree of evolutionary conservation gives us confidence that humans may also respond to MR in a similar manner. Indeed, protein and methionine restriction diets have been associated with beneficial health effects in humans [5], [54] Finding the mechanisms through which this association takes place is important to delineate specific interventions that can mimic DR without its heavy demands. Here we found a sulfur amino acid/adiponectin/DICER axis in adipose tissue that plays an important role in the way DR promotes browning and increases lifespan. This axis represents a promising target for interventions designed to mimic DR and promote beneficial metabolic effects.
Author contribution
B.A.G. performed the experiments with mice and cells. B.B.B. performed the nutrient screen in cells. F.C.G.R. helped with the mouse cohorts. S.S.P. designed, performed and interpreted the worm lifespan assays. E.A.D-S. and W.G.S. designed, performed and interpreted the dcr-1 reporter assays. T.M.B. conducted the protein restriction protocol. V.C-S. performed the HPLC assays. V.D’A., B.A.C., A.A, W.T.F. and M.A.M. provided reagents and infrastructure. M.A.M., supervised the study. B.A.G. and M.A.M. designed the experiments, interpreted the data and wrote the manuscript.
Acknowledgements
We thank Elzira Elisabeth Saviani and Emanoel Cabral for valuable technical support. We thank the National Institute of Science and Technology on Photonics Applied to Cell Biology (INFABIC) at the Universidade Estadual de Campinas to provide access to microscopes, the Caenorhabditis Genetics Center (CGC) for worms and Dr. Amy Pasquinelli for the dcr-1 RNAi clone. CGC is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Carmen Perrone for sharing the composition of the methionine restriction diet, for valuable discussion and for sharing samples of rats exposed to methionine restriction. This study was funded by grants of the Fundação de Amparo à Pesquisa do Estado de São Paulo (2017/01184-9, 2017/07975-8, 2017/22057-5, 2015/03292-8, 2012/07259-7, 2016/02207-0, 2010/52557-0, 2015/01316-7, 2012/50558-5 and 2015/19530-5), Conselho Nacional de Desenvolvimento Científico e Tecnológico (305069/2015-2 and 304995/2014-2) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - German Academic Exchange Service (PROBRAL - 88887.143923/2017-00).
Footnotes
Appendix ASupplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.08.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article: