- Journal List
- HHS Author Manuscripts
- PMC5970038

The Rbm38-p63 Feedback Loop Is Critical for Tumor Suppression and Longevity
Yuqian Jiang
1Comparative Oncology Laboratory, University of California at Davis, Davis, CA
Enshun Xu
1Comparative Oncology Laboratory, University of California at Davis, Davis, CA
Jin Zhang
1Comparative Oncology Laboratory, University of California at Davis, Davis, CA
Mingyi Chen
3Department of Pathology, University of Texas Southwestern Medical Center, Dallas, TX
Elsa Flores
4Department of Molecular Oncology, Moffitt Cancer Center, Tampa, FL
Xinbin Chen
1Comparative Oncology Laboratory, University of California at Davis, Davis, CA
Associated Data
Abstract
The RNA-binding protein Rbm38 is a target of p63 tumor suppressor and can in turn repress p63 expression via mRNA stability. Thus, Rbm38 and p63 form a negative feedback loop. To investigate the biological significance of the Rbm38-p63 loop in vivo, a cohort of WT, Rbm38−/−, TAp63+/− and Rbm38−/−;TAp63+/− mice were generated and monitored throughout their lifespan. While mice deficient in Rbm38 or TAp63 alone died mostly from spontaneous tumors, compound Rbm38−/−;TAp63+/− mice had an extended lifespan along with reduced tumor incidence. We also found that loss of Rbm38 markedly decreased the percentage of liver steatosis in TAp63+/− mice. Moreover, we found that Rbm38 deficiency extends the lifespan of tumor-free TAp63+/− mice along with reduced expression of senescence-associated biomarkers. Consistent with this, Rbm38−/−;TAp63+/− MEFs were resistant, whereas Rbm38−/− or TAp63+/− MEFs were prone, to cellular senescence. Importantly, we showed that the levels of inflammatory cytokines (IL17D and Tnfsf15) were significantly reduced by Rbm38 deficiency in senescence-resistant Rbm38−/−;TAp63+/− mouse livers and MEFs. Together, our data suggest that Rbm38 and p63 function as intergenic suppressors in aging and tumorigenesis and that the Rbm38-p63 loop may be explored for enhancing longevity and cancer management.
Introduction
p63 (1, 2), a member of the p53 family, is expressed as two isoforms, TAp63 and ΔNp63, through P1 and P2 promoters, respectively. TAp63 contains an N-terminal activation domain conserved in p53 and regulates an array of genes for tumor suppression (3). In contrast, our previous study showed that ΔNp63 contains a unique activation domain (4, 5), which is distinct from that in TAp63 and consists of 14 unique N-terminal residues and the proline-rich domain. The biological function of p63 has been elucidated in several mouse models. For example, mice deficient in all p63 isoforms show severe development defects, including absence of skin, teeth, mammary gland and limb, and die soon after birth (3, 6). Interestingly, mice deficient in ΔNp63 isoforms also die soon after birth due to the developmental defects, such as truncated forelimbs and the absence of hind limbs, which largely phenocopies the total p63-null mice (7). By contrast, mice deficient in TAp63 are born alive, but prone to accelerated aging and spontaneous tumors (8, 9). In addition, these mice have defects in lipid metabolism and glucose tolerance (10). Together, these in vivo studies indicate a critical role of p63 in skin development, aging, metabolism, and tumorigenesis.
Rbm38, also called RNPC1, is a RNA-binding protein and contains a highly conserved RNA recognition motif (RRM), which consists of two sub-motifs, RNP1 and RNP2. The RRM motif in Rbm38 is found to share a sequence similarity with the ones found in HuR and nucleolin. Previous work from our lab showed that Rbm38 is a target of the p53 family, including p63 (11, 12). Recently, we found that Rbm38 inhibits p63 expression via binding to the p63 3′ untranslated region (3′UTR) and thus, forms a feedback regulatory loop with p63 (13). Interestingly, like TAp63-deficient mice (8), we found that Rbm38-null mice are prone to premature aging and spontaneous tumors (14). Thus, to investigate the biological significance of the Rbm38-p63 loop in vivo, we generated compound Rbm38−/−;TAp63+/− mice and found that these mice had longer lifespan along with reduced tumor incidence as compared to Rbm38−/− or TAp63+/− mice. Furthermore, we found that the decreased susceptibility of compound Rbm38−/−;TAp63+/− mice to premature aging and spontaneous tumors is at least in part via decreased senescence-associated secretory phenotype (SASP).
Results
Loss of Rbm38 extends the lifespan, and reduces the tumor penetrance, in TAp63+/− mice
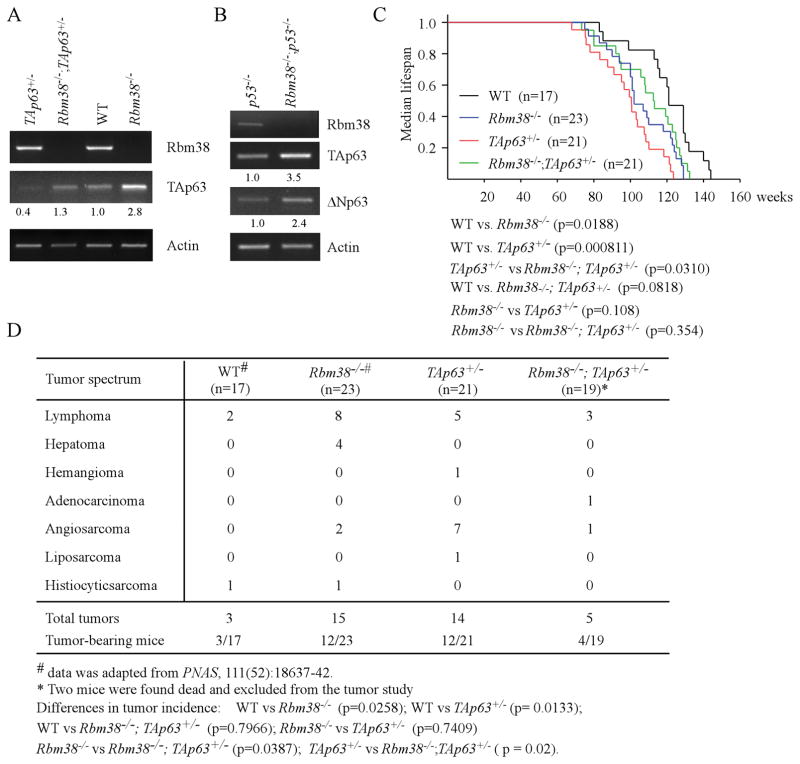
To make sure that the regulation of p63 by Rbm38 in human cells is conserved in murine cells, we generated a cohort of wild-type, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− MEFs. We showed that the level of TAp63 transcripts was increased by loss of Rbm38 in both wild-type and TAp63+/− MEFs (Fig. 1A). To verify this, p53−/− and Rbm38−/−;p53−/− MEFs were generated and confirmed by genotyping (Supplemental Fig. S1A–B). We showed that the level of p63 transcripts was increased in Rbm38−/−;p53−/− MEFs as compared to that in p53−/− MEFs (Fig. 1B). These data indicate that the regulation of p63 by Rbm38 is conserved in murine cells and independent of p53, consistent with our previous observation (13).
(A) The level of Rbm38, TAp63, and actin was determined in WT, Rbm38−/−, TAp63+/−, and Rbm38−/−; TAp63+/− MEFs. The level of TAp63 transcripts was normalized to that of actin and arbitrarily set at 1.0 in WT MEFs. The relative -fold change of each protein level is shown below each lane. The data are representative of three independent experiments. (B) The level of Rbm38, TAp63, ΔNp63, and actin was determined in p53−/− and Rbm38−/−;p53−/− MEFs. (C) Kaplan-Meier survival curves of WT (n=17), Rbm38−/− (n=23), TAp63+/− (n=21), and Rbm38−/−;TAp63+/− (n=21) mice. The median survival time is 121 weeks for WT mice, 102 weeks for Rbm38−/− mice, 101 weeks for TAp63+/− mice, and 113 weeks for Rbm38−/−;TAp63+/− mice. (D) The tumor spectrum in WT (n =17), Rbm38−/− (n=23), TAp63+/− (n=21), and Rbm38−/−;TAp63+/− (n=19) mice.
Rbm38 and p63 form a feedback regulatory loop (13). In addition, mice deficient in Rbm38 or TAp63 are prone to spontaneous tumors (8, 14). Thus, examining the role of the Rbm38-p63 loop in tumorigenesis would be instrumental to understand the biological functions of Rbm38 and/or TAp63. To address this, a cohort of wild-type, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− mice was generated simultaneously and monitored throughout their lifespan. Both WT and Rbm38−/− mice have been analyzed for another tumor study (14) (Supplemental Table S1–2) and were used as controls for current study. As shown in Fig. 1C, the median lifespan was 121 weeks for WT mice, 102 weeks for Rbm38−/− mice, 101 weeks for TAp63+/− mice, and 113 weeks for Rbm38−/−;TAp63+/− mice. Statistical analyses indicated that the median survival time for Rbm38−/− and TAp63+/− mice was significantly shorter than that for WT mice (p=0.0188 for WT vs. Rbm38−/−; p=0.000811 for WT vs. TAp63+/−). Interestingly, the median survival time for Rbm38−/−;TAp63+/− mice was significantly longer than that for TAp63+/− mice (p=0.0310 by LogRank test). Additionally, there was no significant difference in the survival time between WT and Rbm38−/−;TAp63+/− mice (p=0.0818 by LogRank test). Next, histology analysis was performed and showed that TAp63+/− and Rbm38−/− mice were prone to spontaneous tumors (Fig. 1D, Table 1, and Supplemental Table S2), consistent with previous reports (8, 14). While Rbm38−/− mice were prone to lymphomas (Fig. 1D and Supplemental Table S1), TAp63+/− mice were prone to both lymphomas and angiosarcomas (Fig. 1D, Table 1, and Supplemental Fig. S1C). Nevertheless, there was no statistical significance in tumor incidence between Rbm38−/− and TAp63+/− mice (p=0.7409 by χ2 test). Surprisingly, Rbm38−/−;TAp63+/− mice were not tumor-prone as compared to Rbm38−/− or TAp63+/− mice (Fig. 1D, Table 1–2, and Supplemental Table S2). The difference in the tumor incidence was statistically significant between Rbm38−/−;TAp63+/− and Rbm38−/− (p=0.0387 by χ2 test) as well as betwen Rbm38−/−;TAp63+/− and TAp63+/− mice (p=0.02 by χ2 test). Additionally, there was no statistical difference in the tumor incidence between WT and Rbm38−/−;TAp63+/− mice (p=0.7966 by χ2 test). Together, these results suggest that the Rbm38 deficiency extends the lifespan and reduces tumor penetrance in TAp63+/− mice.
Table 1
Survival time and tumor spectrum in TAp63+/− mice (n=21)
| ID | Gender | Survival (Week) | Tumor | Liver Steatosis | Other abnormalities |
|---|---|---|---|---|---|
| 11 | F | 78 | hemangioma | Yes | None |
| 13 | M | 121 | angiosarcoma | Yes | EMH, interstitial nephritis |
| 14 | M | 99 | lymphoma; angiosarcoma | No | EMH, interstitial nephritis |
| 16 | M | 124 | angiosarcoma | No | spleen with white pulp hyperplasia |
| 17 | M | 103 | B-cell lymphoma; angiosarcoma | No | interstitial nephritis |
| 25 | M | 118 | angiosarcoma | No | spleen with red pulp hyperplasia |
| 28 | M | 95 | liposarcoma | Yes | interstitial nephritis |
| 5 | F | 108 | lymphoma | No | interstitial nephritis |
| 3 | F | 122 | lymphoma | No | spleen with white pulp hyperplasia, interstitial nephritis |
| 1-20-1 | M | 91 | angiosarcoma | Yes | red pulp hemorrhage |
| 10-2-1 | M | 83 | lymphoma | Yes | red pulp hemorrhage |
| 11-15-2 | F | 75 | hemangiosarcoma | Yes | white pulp hyperplasia, glomerulosclerosis |
| 19 | F | 97 | No | No | interstitial nephritis |
| 10 | M | 88 | No | No | interstitial nephritis |
| 10-2-4 | M | 110 | No | Yes | red pulp hemorrhage |
| 3-2-15 | F | 108 | No | Yes | EMH, white pulp hyperplasia |
| 21 | M | 101 | No | No | penomonia, interstitial nephritis, glomerulosclerosis |
| 11-15-3 | M | 104 | No | Yes | None |
| 11-19-1 | M | 76 | No | Yes | None |
| 2-13-6 | F | 68 | No | No | None |
| 27 | M | 101 | No | Yes | None |
|
| |||||
| Used for experiments below | |||||
|
| |||||
| 2-13-6 | M | 68 | Fig. 3C–D, Fig. 4D–E | ||
| 2-13-1 | M | 113 | Fig. 3C–D, Fig. 4D–E | ||
| 24 | M | 120 | Supplemental Fig. S3A | ||
EMH: extramedullary hematopoiesis
Table 2
Survival time and tumor spectrum in Rbm38−/−; TAp63+/− mice (n=21)
| ID | Gender | Survival (week) | Tumor | Liver steatosis | Other abnormalities |
|---|---|---|---|---|---|
| 8-17-3 | M | 127 | lymphoma | No | Red pulp hemorrhage in spleen |
| 8-19-9 | F | 125 | lymphoma | No | Interstitial nephritis |
| 8-19-8 | M | 113 | hemangiosarcoma, adenocarcinoma | Yes | None |
| 10-6-2 | F | 106 | lymphoma | Yes | None |
| 5-1-12 | M | 128 | No | No | None |
| 5-1-6 | M | 108 | No | No | None |
| 6-15-9 | M | 132 | No | No | None |
| 8-11-3 | F | 94 | No | Yes | EMH |
| 8-15-1 | M | 113 | No | No | None |
| 11-20-3 | M | 80 | No | No | Red pulp hemorrhage in spleen, interstitial nephritis |
| 5-4-4 | F | 80 | No | No | White pulp hyperplasia in spleen |
| 10-21-6 | M | 131 | No | No | None |
| 11-23-2 | M | 126 | No | No | None |
| 11-26-4 | M | 121 | No | No | Red pulp hemorrhage in spleen |
| 11-28-6 | M | 95 | No | No | None |
| 5-1-3 | M | 92 | No | No | None |
| 7-28-5 | F | 73 | No | No | Liver ballooning degeneration |
| 8-11-4 | M | 125 | No | No | None |
| 11-2-4 | M | 108 | No | No | None |
| 8-20-4 | M | 123 | N/A | N/A | Found dead |
| 10-2-5 | M | 119 | N/A | N/A | Found dead |
|
| |||||
| Used for experiments below | |||||
|
| |||||
| 11-2-2 | M | 83 | Fig. 3C–D, Fig. 4D–E | ||
| 11-26-4 | M | 113 | Fig. 3C–D, Fig. 4D–E | ||
| 5-1-3 | M | 92 | Supplemental Fig. S3B | ||
EMH: extramedullary hematopoiesis
Rbm38 deficiency reduces the incidence of liver steatosis in TAp63+/− mice
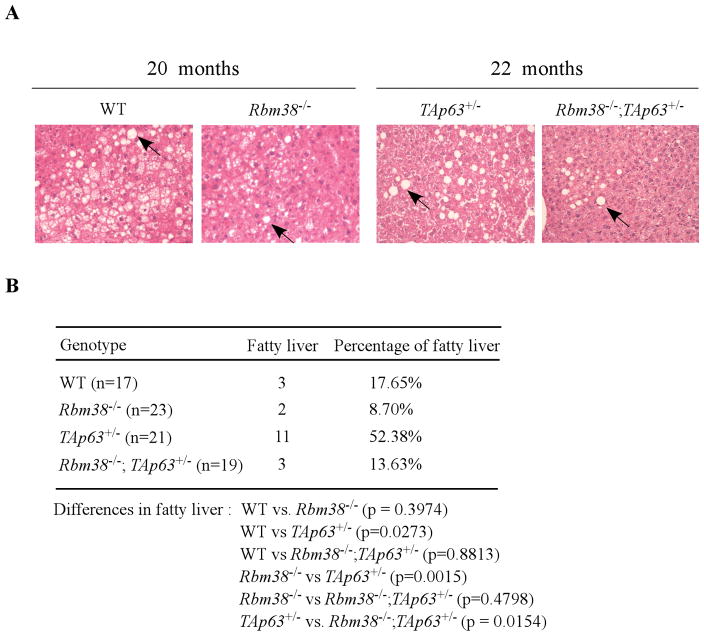
TAp63 plays a role in lipid metabolism and as a result, mice deficient in TAp63 are prone to liver steatosis (fatty liver disease) (10), a process of abnormal retention of lipids in liver cells. Thus, liver tissues of TAp63+/−, and Rbm38−/−;TAp63+/− mice along with WT and Rbm38−/− mice were examined for liver steatosis. Although WT and Rbm38−/− mice were analyzed for a previous tumor study (14), liver steatosis was not examined in these mice. We found that all four groups of mice developed liver steatosis (Fig. 2A), which is characterized by the large vacuoles or “bubbles” as fat solves during tissue processing. Interestingly, the percentage of liver steatosis was low in WT (17.65%) and Rbm38−/− (8.7%) mice, but high in TAp63+/− (52.38%) mice (Fig. 2B). Surprisingly, we found that Rbm38 deficiency markedly decreased the incidence of liver steatosis from 52.38% in TAp63+/− mice to only 13.63% in Rbm38−/−;TAp63+/− mice (Fig. 2B). Pairwise comparison indicated that the difference in liver steatosis was significant between WT and TAp63+/− mice (p=0.0273 by χ2 test), between Rbm38−/− and TAp63+/− mice (p=0.0015 by χ2 test), and between Rbm38−/−;TAp63+/− and TAp63+/− mice (p=0.0154 by χ2 test). By contrast, there was no significant difference in liver steatosis between WT and Rbm38−/− mice (p=0.3974 by χ2 test), between WT and Rbm38−/−;TAp63+/− mice (p=0.8813 by χ2 test), and between Rbm38−/− and Rbm38−/−;TAp63+/− mice (p=0.4798 by χ2 test). Together, these data suggest that Rbm38 deficiency ameliorates liver steatosis in TAp63+/− mice.
(A) H&E stained liver sections from WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− mice at age of 20–22 months. Arrows indicate the liver steatosis in the liver tissues characterized as large vacuoles in the liver tissue. (B) The percentage of liver steatosis in WT (n=17), Rbm38−/− (n=23), TAp63+/− (n=21), and Rbm38−/−;TAp63+/− (n=19) mice.
Loss of Rbm38 alleviates aging-related phenotypes in TAp63+/− mice
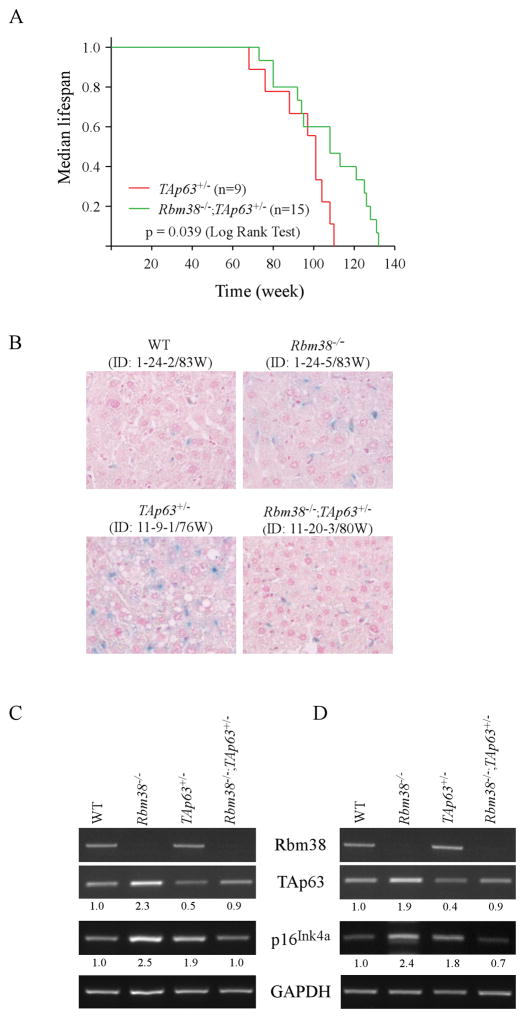
Previous studies indicate that mice deficient in TAp63 or Rbm38 exhibited aging-related phenotypes such as shorter lifespan and reduced body fat (9, 14). Thus, to determine the role of the Rbm38-p63 loop in accelerated aging, the lifespan of tumor-free TAp63+/−, Rbm38−/−, and Rbm38−/−;TAp63+/− mice were plotted. Interestingly, we found that the median lifespan was 101 weeks for TAp63+/− mice, 94 weeks for Rbm38−/− mice, and 108 weeks for Rbm38−/−;TAp63+/− mice (Fig. 3A and supplemental Fig. S2A–B). The difference in lifespan between Rbm38−/−;TAp63+/− and TAp63+/− mice was statistically significant (p=0.039 by LogRank test) (Fig. 3A). However, there was no significant difference in the lifespan between Rbm38−/−;TAp63+/− and Rbm38−/− mice (p=0.138 by LogRank test) (Supplemental Fig. S2A) or between TAp63+/− and Rbm38−/− mice (p=0.728 by LogRank test) (Supplemental Fig. S2B). Next, the activity of senescence associated-β-galactosidase (SA-β-gal), a widely used biomarker for aging (15, 16), was examined in the liver tissues of gender-matched WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− mice at age of around 83 weeks (Fig. 3B). The WT and Rbm38−/− mice were generated from a previous study (Supplemental Tables S1–2) (14). We found that the SA-β-gal activity was enhanced in Rbm38−/− and TAp63+/− livers as compared to that in WT livers (Fig. 3B). Importantly, in Rbm38−/−;TAp63+/− livers, the SA-β-gal activity was markedly reduced when compared to that in Rbm38−/− and TAp63+/− livers but was similar to that in WT livers (Fig. 3B). Similar observations were found in another set of age- and gender-matched WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− mice (Supplemental Fig. S3A). We also showed that the SA-β-gal activity was reduced in the liver and kidney tissues of a Rbm38−/−;TAp63+/− mouse as compared to TAp63+/− mouse (Supplemental Fig. S3B). Furthermore, the level of p16Ink4a transcript, an aging biomarker (17, 18), was examined in liver tissues of WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− mice at age of around 83 weeks (Fig. 3C) or 124 (Fig. 3D) weeks. We showed that Rbm38 deficiency led to an increase in TAp63 transcript in Rbm38−/− and Rbm38−/−;TAp63+/− liver tissues (Fig. 3C), consistent with previous observations (13) (Fig. 1A). Notably, compared to WT livers, the level of p16Ink4a transcript was increased in the Rbm38−/− and TAp63+/− livers, but unchanged or slightly decreased in Rbm38−/−;TAp63+/− livers (Fig. 3C–D, p16Ink4a panels). Similarly, we showed that the level of liver p16Ink4a transcripts in a Rbm38−/−;TAp63+/− mouse was lower than that in a TAp63+/− mouse (Supplemental Fig. 3C). Together, these data suggest that Rbm38 deficiency alleviates aging-related phenotypes in TAp63+/− mice.
(A) Kaplan-Meier survival curves of tumor-free TAp63+/− (n=9) and Rbm38−/−;TAp63+/− (n=15) mice. The median survival time is 101 weeks for TAp63+/− mice and 108 weeks for Rbm38−/−;TAp63+/− mice (p=0.039 by LogRank test). (B) The SA-β-gal-stained liver tissues of WT (ID: 1-24-2/83W), Rbm38−/− (ID:1-24-5/83W), TAp63+/− (ID: 11-9-1/76W), and Rbm38−/−;TAp63+/− (ID: 11-20-3/80W) were sectioned and counterstained with nuclear fast red. (D) The level of Rbm38, TAp63, p16Ink4A and GAPDH was determined in the liver tissues from WT (ID: 1-24-2/83W), Rbm38−/− (ID:1-24-5/83W), TAp63+/− (ID:2-13-6/68W) and Rbm38−/−;TAp63+/− (ID:11-2-2/83W) mice. (E) The level of Rbm38, TAp63, p16Ink4A and GAPDH was determined in the liver tissues from WT (ID: 3-9-7/128W), Rbm38−/− (ID: 11-16-14/129W), TAp63+/− (ID: 2-13-1/113W) and Rbm38−/−;TAp63+/− (ID:11-26-4/124W) mice.
Loss of Rbm38 reduces cellular senescence in TAp63 heterozygous MEFs and liver tissues
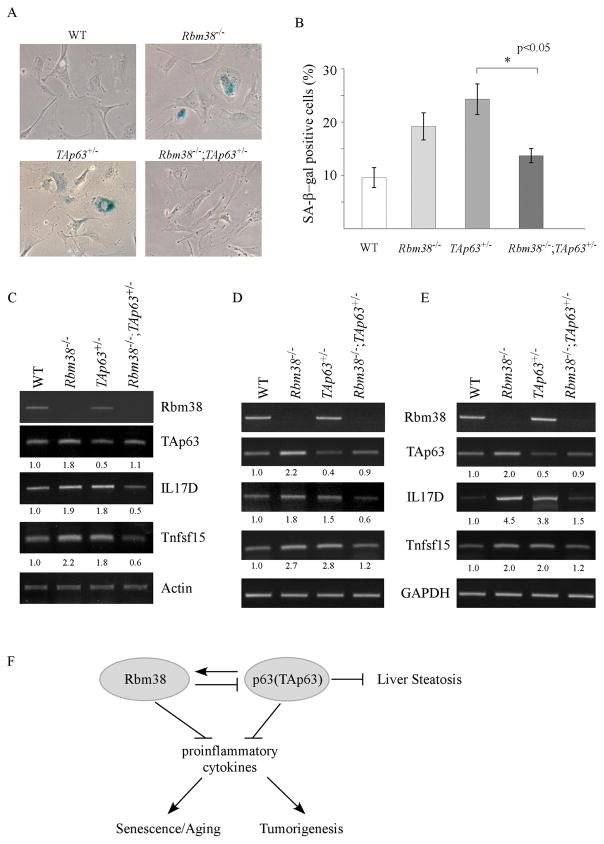
Cellular senescence serves as an important tumor-suppressor mechanism by preventing the proliferation of premalignant cells (19, 20). Interestingly, recent studies suggest that cellular senescence serves as a double-edged sword in promoting aging and cancer as senescent cells secrets many proinflammatory cytokines, a process called SASP (21, 22). Thus, SA-β-gal assay was performed in WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− MEFs. We found that the percentage of SA-β-gal-positive cells was markedly increased in Rbm38−/− MEFs (20%) and TAp63+/− MEFs (25%) as compared to that in wild-type MEFs (10%) (Fig. 4A–B). Importantly, SA-β-positive cells were markedly reduced by loss of Rbm38 from 25% in TAp63+/− MEFs to 13% in Rbm38−/−;TAp63+/− MEFs, which was statistically significant (p<0.05) (Fig. 4A–B). Previously, we showed that Rbm38 deficiency leads to cellular senescence (23) along with increased expression of IL17D and Tnfsf15 (14). IL17D, a member of IL17 family, and Tnfsf15, a member of a member of the tumor necrosis factor (TNF) ligand superfamily, are proinflammatory cytokines (24, 25) and likely involved in SASP. Thus, RT-PCR was performed to measure the levels of IL17D and Tnfsf15 in WT, Rbm38−/−, TAp63+/− and Rbm38−/−;TAp63+/− MEFs. As a control, we measured the level of TAp63 transcript and showed that Rbm38 deficiency led to an increase in TAp63 transcript in Rbm38−/− and Rbm38−/−;TAp63+/− MEFs (Fig. 4C), consistent with a previous report (13). Similarly, the levels of IL17D and Tnfsf15 were increased in Rbm38−/− MEFs (Fig. 4C), consistent with a previous report (14). Interestingly, we found that the levels of IL17D and Tnfsf15 transcripts were also increased in TAp63+/− MEFs as compared to that in wild-type MEFs (Fig. 4C). However, the levels of IL17D and Tnfsf15 were reduced by Rbm38 deficiency in Rbm38−/−;TAp63+/− MEFs (Fig. 4B). To further test this, the levels of IL17D and Tnfsf15 transcripts were examined in the liver tissues of WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− mice at age of around 83 weeks (Fig. 4D) or 124 weeks (Fig. 4E). We found that the levels of IL17D and Tnfsf15 transcripts were increased in the Rbm38−/− and TAp63+/− liver tissues as compare to WT liver tissues (Fig. 4D–E). By contrast, in Rbm38−/−;TAp63+/− livers, the level of IL17D and Tnfsf15 transcripts was similar to that in WT liver tissues, but much lower than that in Rbm38−/− and TAp63+/− liver tissues (Fig, 4D–E). Together, these data suggest that the Rbm38-p63 loop modulates tumor suppression and premature aging potentially via SASP.
(A) Primary WT, Rbm38−/−, TAp63+/− and Rbm38−/−;TAp63+/− MEFs at passage 4 were used for SA-β-gal assay as described in the Materials and Methods. (B) Quantification of the percentage of SA- β-gal-positive cells shown in (A). (C) The level of Rbm38, TAp63, IL17D, Tnfsf15 and actin was determined in WT, Rbm38−/−, TAp63+/−, and Rbm38−/−;TAp63+/− MEFs. (D) The level of IL17D, Tnfsf15 and GAPDH was determined in the liver tissues from WT (ID: 1-24-2/83W), Rbm38−/− (ID: 1-24-5/83W), TAp63+/− (ID: 2-13-6/68W) and Rbm38−/−;TAp63+/− (ID:11-2-2/83W) mice. (E) The level of IL17D, Tnfsf15 and GAPDH was determined in the liver tissues from WT (ID: 3-9-7/128W), Rbm38−/− (ID: 11-16-14/129W), TAp63+/− (ID: 2-13-1/113W) and Rbm38−/−;TAp63+/− (ID:11-26-4/124W) mice. (F) A proposed model for the role of Rbm38-p63 loop in inflammation, aging and tumorigenesis.
Discussion
We showed previously that Rbm38 and p63 mutually regulate each other and forms a feedback loop (11, 13). Thus, to investigate the Rbm38-p63 loop in vivo, a cohort of WT, Rbm38−/−, TAp63+/−, Rbm38−/−;TAp63+/− mice was generated and monitored for aging and spontaneous tumors. Interestingly, we found that compared with Rbm38−/− or TAp63+/− mice, compound Rbm38−/−;TAp63+/− mice had a longer lifespan and decreased susceptibility to spontaneous tumors and liver steatosis (Figs. 1–2). We also showed that cellular senescence, a key mediator of aging and cancer, was alleviated by loss of Rbm38 in Rbm38−/−;TAp63+/− MEFs and tissues. Furthermore, the levels of proinflammatory cytokines (IL17D and Tnfsf15) were markedly decreased by loss of Rbm38, thus relieving the burden of inflammation in Rbm38−/−;TAp63+/− mice. Based on these findings, a model for the role of the Rbm38-p63 loop in longevity and tumor suppression is proposed and shown in Fig. 4F.
Here, we found that Rbm38−/−;TAp63+/− mice exhibited significant improvements in longevity and tumor suppression as compared to TAp63+/− mice, (Fig. 1C–D). Similarly, the median survival time for tumor-free Rbm38−/−;TAp63+/− mice was significantly longer than that for TAp63+/− mice (Fig. 3A). These data suggest that the aging and tumorigenesis mediated by TAp63 deficiency can be ameliorated by Rbm38 deficiency, representing a phenomenon called “intergenic suppression”. Thus, to explore how TAp63 and Rbm38 act as intergenic suppressors in aging and tumorigenesis, three possibilities merit further investigation: (1) the increased level of TAp63 in Rbm38−/−;TAp63+/− mice (Figs. 1A–B, 3C–D, and 4C–E) may contribute to the longer lifespan and reduced tumor penetrance; (2) loss of Rbm38 may alter a pathway that compensates for the TAp63 pathway in tumor suppression and premature aging; (3) IL17D and Tnfsf15 are suppressed by combined loss of Rbm38 and TAp63 (Fig. 4C–E), which subsequently reduces the burden of inflammation necessary for aging and tumor promotion.
In this study, we also found that the percentage of mice with liver steatosis was significantly reduced in Rbm38−/−;TAp63+/− mice as compared to that in TAp63+/− mice (Fig. 2B). We postulate that the increased expression of TAp63 by Rbm38 deficiency may be responsible for the decreased liver steatosis in compound Rbm38−/−;TAp63+/− mice (Fig. 1A–B). Indeed, TAp63 is a major regulator in lipid metabolism as TAp63-deficient mice become obese and prone to liver steatosis (10). However, a recent study indicated that activation of TAp63 promotes, whereas down-regulation of TAp63 inhibits, liver steatosis via IKKβ/ER stress in a p53-deficient mouse model (26). Another possibility is probably due to the reduction of adipose tissue induced by Rbm38 deficiency (14), which would be independent of p63. Thus, further studies are needed to determine how the Rbm38-p63 loop regulates lipid metabolism by using conditional Rbm38- and/or TAp63-deficient mouse models.
Cellular senescence has been considered as a potent tumor-suppressive mechanism by inducing growth arrest of malignant cells (27, 28). However, recent studies suggest that senescent cells can secret proinflammatory cytokiens, which would change the tissue microenvironment and promote tumorigenesis (21, 29). Based on these observations, it is hypothesized that cellular senescence suppresses tumor initiation but promotes tumor progression (30). In our study, we found that cellular senescence and two proinflammatory cytokines (IL17D and Tnfsf15) were reduced in Rbm38−/−;TAp63+/− MEFs and tissues as compared to that in Rbm38−/− or TAp63+/− MEFs and tissues (Figs. 3–4). Interestingly, IL17D can stimulate the production of IL6 and IL8, both of which are critical SASP factors (31). Additionally, Tnfsf15 is known to activate NF-kappaB, which is a key regulator of SASP (32). Thus, our results suggest that SASP is alleviated by loss of Rbm38, leading to the extended lifespan and reduced tumor penetrance in Rbm38−/−;TAp63+/− mice. However, it is not clear how Rbm38 and/or TAp63 control IL17D and Tnfsf15 expression. Additionally, it is not clear how IL17D and Tnfsf15 are involved in TAp63- and/or Rbm38-mediated longevity and tumor suppression. The answers to these questions would help us better understand the biological significance of the Rbm38-p63 loop in tumor suppression and premature aging.
Materials and Methods
Mice
Rbm38-conditional knockout mice (on a pure C57BL/6 background) were previously generated as described in (23). The TAp63+/− mice (on a C57BL/6 background) were previously generated by Dr. Elsa Flores’ laboratory (9). Rbm38−/−; TAp63+/− mutant mice were generated by intercrossing Rbm38+/− with Rbm38+/−; TAp63+/− mice. All animals and use protocols were approved by the University of California at Davis Institutional Animal Care and Use Committee.
MEF isolation
To isolate WT, Rbm38−/−, TAp63+/−, and Rbm38−/−; TAp63+/− MEFs, Rbm38+/−;TAp63+/− mice were bred with Rbm38+/−;TAp63+/− mice. At 13.5-day, embryos were isolated as described previously (33). The MEFs were cultured in DMEM supplemented with 10% FBS (HyClone), 55 μM β-mercaptoethanol, and 1× non-essential amino acids (NEAA) solution (Cellgro).
RNA Isolation and RT-PCR Analysis
The RT-PCR analysis was performed as previously described (34). Briefly, total RNA was isolated and then subjected to cDNA synthesis using RevertAid Reverse transcriptase (Fisher). The PCR program used for amplification was (i) 95 °C for 5 min, (ii) 95 °C for 1 min, (iii) 58 °C for 1min, (iv) 72 °C for 1 min, and (v) 72 °C for 10 min. From steps ii–iv, the cycle was repeated 22 times for actin or GAPDH, 30 times for Rbm38 and TAp63, 35 times for IL17D, Tnfsf15 and p16. The primers for mouse TAp63 were a forward primer, 5′-TAG AGA TCT GCC ATG TCG CA-3′, and a reverse primer, 5′-GCA TGC GGA TAC AAT CCA TG-3′. The primers for Rbm38, actin, IL17D, Tnfsf15, GAPDH, and p16 were the same as the ones described previously (14).
SA-β-Gal Staining
The senescence assay was performed as described previously (35). Briefly, primary MEFs at passage 4 were harvested, fixed, and stained with X-gal overnight at 37°C. The percentage of SA-β-gal-positive cells was calculated as the ratio of SA-β-gal-positive cells versus total cells. Total 150 cells were counted in triplicates. For SA-β-Gal staining in tissues, tissues were fixed with 2% (vol/vol) formaldehyde and 0.2% glutaraldehyde for 20 min at room temperature, followed by staining with fresh β-gal staining solution overnight at 37 °C. The tissues were processed, embedded with paraffin, counterstained with nuclear fast red.
Histological Analysis
The histological assay was performed as previously described (14). Briefly, mouse tissues were fixed with formalin for 18 hour, followed by embedding process in paraffin blocks. Tissue sections (6 μm) were sectioned and stained with Hematoxylin and eosin. All the mouse tissues were blindly reviewer by a pathologist without knowing the mouse genotypes.
Statistical Analysis
The LogRank Test was used for determination of the differences in survival of different genotypes. χ2 test was used for comparison between tumor incidence or liver steatosis from different genotypes. Student t-test was used to determine the statistical significance of SA-β-gal staining assay. A p value that is smaller than 0.05 was considered significant.
Acknowledgments
This work is supported in part by National Institutes of Health (CA195828 to Chen, XB).
Footnotes
Conflict of Financial Interest: nothing to disclose
Author contribution: Y.J, E.X., and J.Z. performed experiments. Y.J., J.Z., X.C. designed and analyzed the data; E.F. provided the TAp63+/− mice. M.C. performed histological analysis. Y.J., J.Z., and X.C. wrote the manuscript.