Mating in the absence of fertilization promotes a growth-reproduction versus lifespan trade-off in female mice
Michael Garratt
aDepartment of Anatomy, School of Biomedical Sciences, University of Otago, Dunedin 9016, New Zealand;
Heather Try
bEvolution and Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia;
Kristina O. Smiley
aDepartment of Anatomy, School of Biomedical Sciences, University of Otago, Dunedin 9016, New Zealand;
cCentre for Neuroendocrinology, University of Otago, Dunedin 9016, New Zealand
David R. Grattan
aDepartment of Anatomy, School of Biomedical Sciences, University of Otago, Dunedin 9016, New Zealand;
cCentre for Neuroendocrinology, University of Otago, Dunedin 9016, New Zealand
Robert C. Brooks
bEvolution and Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia;
Author contributions: M.G. and R.C.B. designed research; M.G., H.T., and K.O.S. performed research; D.R.G. contributed new reagents/analytic tools; M.G. and R.C.B. analyzed data; and M.G. wrote the paper.
Associated Data
- Data Availability Statement
All pertinent data are available on the Open Science Network (https://osf.io/y49p2/).
Significance
Pregnancy and lactation are energetically costly and can have negative effects for later-life reproduction and lifespan. To become pregnant females must mate, and these mating interactions with males could also have long-term effects on female life history. Evidence supporting this prediction has been lacking until now. Here we show that allowing female mice to mate early in life, with only sterilized males, permanently affects female life history and aging. Mated females gain and maintain body weight across life, have a greater late-life reproductive output, but suffer a shorter lifespan. These responses explain some trade-offs previously attributed to pregnancy and lactation and highlight that exposure to sexual stimuli alone can have a major effect on female life history in mammals.
Abstract
Trade-offs between growth, reproduction, and lifespan constrain animal life histories, leading to evolutionary diversification of life history cycles in different environments. In female mammals, gestation and lactation are expected to impose the major costs of reproduction, driving reproductive trade-offs, although mating also requires interactions with males that could themselves influence life history. Here we show that a male’s presence by itself leads to lifelong alterations in life history in female mice. Housing C57BL/6J female mice with sterilized males early in life led to an increase in body weight, an effect that persisted across life even when females were later allowed to produce pups. We found that those females previously housed with sterile males also showed enhanced late-life offspring production when allowed to reproduce, indicating that earlier mating can influence subsequent fecundity. This effect was the opposite to that seen in females previously housed with intact males, which showed the expected trade-off between early-life and late-life reproduction. However, housing with a sterile male early in life came at a cost to lifespan, which was observed in the absence of females ever undergoing fertilization. Endocrinologically, mating also permanently reduced the concentration of circulating prolactin, a pituitary hormone influencing maternal care. Changes in hormone axes that influence reproduction could therefore help alter life history allocation in response to opposite-sex stimuli. Our results demonstrate that mating itself can increase growth and subsequent fecundity in mammals, and that responses to sexual stimuli could account for some lifespan trade-offs normally attributed to pregnancy and lactation.
Reproduction is costly and can trade-off against lifespan, subsequent reproduction, and late-life physical function (1, 2). The dramatic energetic demands of gestation and lactation experienced by mammalian females represent some of the most obvious costs of reproduction (3, 4). Energy and nutrients provided via the placenta to the fetus or via milk to the neonate can no longer be used for other functions, such as somatic maintenance or future reproduction. While energetically costly, the life history effects of pregnancy and lactation on aging and lifespan cannot normally be distinguished from effects of mating, or of mere exposure to males, since this exposure occurs concurrently with fertilization. Here we tease apart the relative effects of parental care from mating and male presence by manipulating the male capacity to impregnate or mate with females, and thus test for the effects of mating/social interactions on life history trade-offs.
There are reasons to believe that social interactions by themselves might influence life history and aging. Social cues have priming effects on reproductive physiology. In mammals, this can influence estrous cycling and the maintenance or loss of pregnancy, depending on the presence of paternal or unfamiliar opposite sex cues (5, 6). Exposure to opposite sex odors alone can also speed up sexual development (7), facilitating a change in early-life reproduction that could have implications for other aspects of life history. While the consequences of this social regulation of reproductive physiology for late-life performance and aging in mammals have been unknown, there are some examples in short-lived invertebrates in which lifespan can be affected by simply the act of mating or pheromone exposure (8–11), highlighting a potential role of social cues in regulating the life course.
To test whether mating itself has effects on life history in mice, we paired females with vasectomized males from 28 to 300 d of age, allowing us to examine the consequences of male presence and mating on subsequent life history without the confounding effect of female pregnancy and lactation (Fig. 1A). Vasectomized males will mate with females and maintain normal androgen levels, but do not produce sperm in their ejaculate (12). We also paired a subsample of females with castrated males, allowing us to assess the effects of housing with nonandrogenized males, which show greatly reduced mating behavior (13) and reduced production of androgen-dependent sexual signals (14). The life history consequences of this opposite-sex exposure were then contrasted with those in females without a male present and in females housed with intact, sham-vasectomized males, which fertilize females and thus induce pregnancy and lactation.
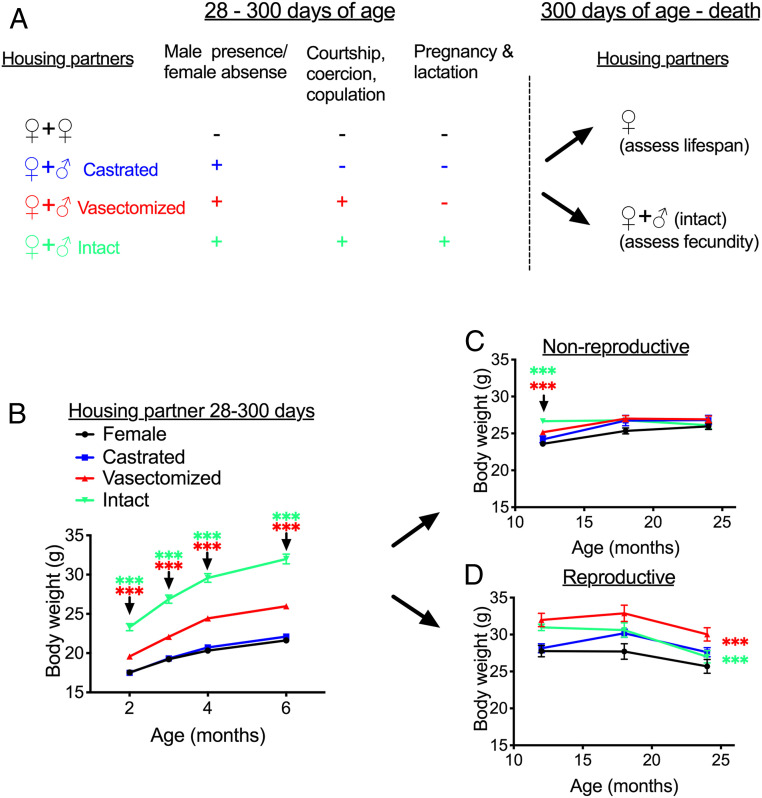
Female body weight in response to early-life and late-life social conditions. (A) Schematic of experimental design and female housing partners at different stages of the experiment. (B) Female weights from age 2 to 6 mo, when females were housed with two females or with a female and a surgically operated male. (C and D) Female body weights from age 12 to 24 mo, when later housed with either another female (nonreproductive) (C) or with another female and a male (reproductive) (D). Data are presented as mean ± SEM. ***P < 0.005 for contrast between females housed/previously housed with vasectomized males and females with other females (red asterisk) or between females previously housed with intact males and those previously housed with females (green asterisk). In D, contrasts are calculated from across the whole time course, while in B and C the contrast was calculated at specific times when the treatment effect differed between groups. n = 54 per housing partner group up to 6 mo, then 18 to 30 per early-life group per time point in the nonreproductive late life-environment and 13 to 23 per early-life group in the reproductive late-life environment.
Results
Early-Life Mating Leads to Life-Long Elevations in Body Weight.
Females housed with different social partners differed in their weight trajectory across the 300-d manipulation period (time × treatment interaction: F9,246 = 7.86, P < 0.001) (Fig. 1B). There were significant overall effects of treatment at each time point after pairing (P < 0.001 from 2 mo), with the difference between groups increasing over time. At age 6 mo, females in different social environments differed in weight (overall effect of group: F3,212 = 160.69, P < 0.001), with females paired with either vasectomized males or intact males substantially heavier than female-female controls (Fig. 1B). For females housed with intact males, this is expected, as females were in various stages of pregnancy and lactation, but females housed with vasectomized males were also 20% heavier than control animals at age 6 mo. In contrast, pairing with castrated males had no effect on body weight compared with female-female controls (P = 0.37).
At age 300 d, the original housing partners were removed. A subset of females from each treatment group were maintained with a female sibling for their remaining lives to assess lifespan, while the others were housed with an intact male to assess the consequences of early-life reproductive environments for late-life reproduction (Fig. 1A). Assessing body weight post-300 d, from age 12 to 24 mo, showed that the effects of earlier social conditions on body weight were maintained to 24 mo of age only when females were kept in a reproductive condition (i.e., housed with intact males), and not when females were housed in a nonreproductive condition (i.e., housed with females) (interaction between early-life and late-life reproductive environments: F3,148 = 4.09, P = 0.008). In the nonreproductive late-life environment, the effect of early life social environments on body weight diminished progressively over time (interaction between pre-300 d treatment and time: F6,182 = 9.44, P < 0.001), with a difference in weight observable between females previously housed in different early-life social conditions at 12 mo but no significant difference at 18 or 24 mo (Fig. 1C). In contrast, when females were subsequently housed with intact males, those previously housed with vasectomized or intact males from 28 to 300 d maintained greater body weight across the remainder of the life span (interaction between pre-300 d treatment and time: F6,114 = 1.17, P = 0.33; effect of pre-300 d treatment on weight from 12 to 24 mo: F3,57 = 8.64, P < 0.001), while previous housing with castrated males had no significant effect on later-life weight when housed with intact males (Fig. 1D). The life-long body weight profiles for each group of females are shown in SI Appendix, Fig. S1.
Early-Life Mating without Fertilization Increases Late-Life Reproductive Capacity.
We assessed the impact of early-life social environments on later-life reproduction in the subset of females housed with intact males post-300 d. Across groups, a similar proportion of females produced at least 1 litter after pairing with intact males (17 of 21 in those previously housed with other females, 14 of 20 in those previously with vasectomized males, 20 of 21 in those previously housed with castrated males, and 19 of 23 in those previously housed with intact males; χ2 test of independence, χ2[3] = 4.53; P = 0.21).
To assess fecundity in females paired with intact males, we analyzed cumulative offspring weaned by those females that reproduced at least once. We fitted generalized linear mixed models (GLMMs) with dam ID as a subject random effect and cage as a random effect, plus treatment as a fixed effect and time since mating started (in 50-d bins) as a fixed covariate. For the cumulative number of offspring weaned over time, there was an overall significant effect of early-life social treatment (F3, 66.12 = 6.26, P = 0.001), but also a significant effect of time (F4, 257.45 = 125.33, P < 0.0001) and a time × treatment interaction (F12, 257.46 = 6.04, P < 0.0001), indicating that females in previous social environments differed in their relative fecundity over time after pairing.
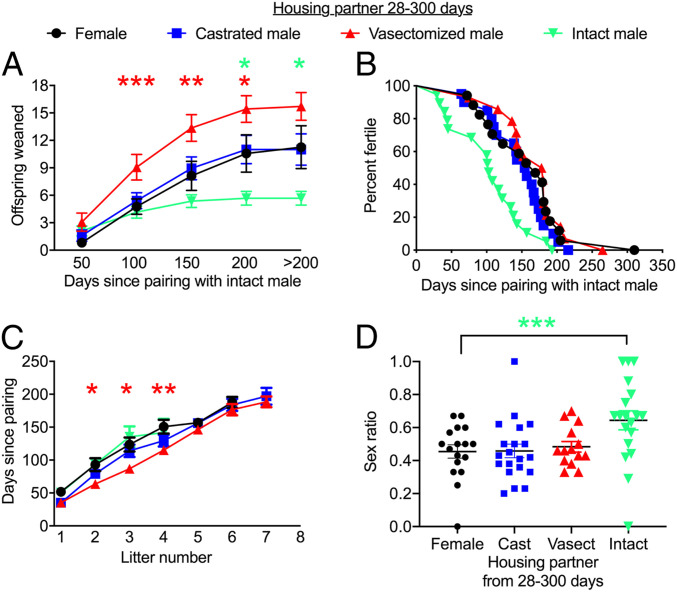
Examining the total number of offspring weaned up to specific time points (i.e., cumulative offspring produced) revealed no overall significant difference in offspring production between treatments at 50 d after pairing (overall treatment effect, P > 0.05), but the total offspring produced differed significantly between groups thereafter (treatment effects, P < 0.05 in each case). This was partly attributable to females that were previously housed with vasectomized males producing offspring quicker, weaning significantly more offspring from 50 to 200 d after pairing than those previously housed with females (Fig. 2A). The interaction between time and treatment was also attributable to an earlier slowdown in offspring production in females previously housed with intact males (Fig. 2A), with these females showing an earlier age at last reproduction compared with controls (log-rank test, overall difference in age at last litter for those housed in different early-life social environments, P = 0.002; contrast between those previously housed with intact males and females, P = 0.009) (Fig. 2B). Thus, interactions with males early in life in the absence of fertilization can enhance later-life fecundity, whereas fertilization itself promotes a trade-off causing earlier reproductive senescence.
Early-life social conditions influence subsequent reproductive success. Females were paired with intact males at age 300 d, and the number of offspring weaned was recorded. (A) Cumulative offspring weaned since pairing. The mean number of offspring produced up to each time point is denoted by each error bar, with >200 d representing the total number of offspring produced. (B) Survival curve plotting age at last litter for different individuals as a function of time from pairing. (C) Time taken to produce a specific number of litters. (D) Sex ratio of total offspring produced by each female, calculated as the number of males weaned divided by the total number of weaned offspring: M/(M + F). Each dot represents the value for a different individual. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.005 at specific time points (or sex ratio in D), for contrast between females previously housed with vasectomized males and females previously housed with other females (red asterisk) or between females previously housed with intact males compared and those previously housed with females (green asterisk).
To understand the causes for these differences in fecundity, we examined litter weight, size, and frequency in females previously housed in different social environments. Litter size and weight were not significantly affected by treatment (SI Appendix, Tables S1 and S2); however, previous social experience had a strong effect on the rate of litter production. Females previously paired with different housing partners produced their first two to four litters at different rates (treatment effect for time to second litter, P = 0.037; time to third litter, P = 0.044; time to fourth litter, P = 0.046). This was a consequence of the faster litter production by females previously paired with vasectomized males (Fig. 2C) compared with females previously housed with other females, although this between-group difference diminished and was nonsignificant after the fourth litter.
We also tested whether previous social environments influenced the sex ratio or weight of pups produced. Previous housing conditions influenced the sex ratio of later litters at weaning (F3,69 = 4.11, P = 0.010) (Fig. 2D). This effect was attributable to the females previously housed with intact males producing more male-biased litters at weaning compared with females previously housed with females, while sex ratios were similar in females from other treatments. Females previously housed with intact males produced fewer female offspring compared with females previously housed with other females (SI Appendix, Fig. S2), leading to the sex ratio difference, while the number of male offspring was not significantly different between these two groups (SI Appendix, Fig. S3). However, we also note that the fewer female offspring produced by those females previously housed with intact males were also slightly heavier at weaning (SI Appendix, Fig. S4), with no weight difference seen in male offspring (SI Appendix, Fig. S5). This highlights a potential change in sex allocation that could influence the fitness consequences of reduced late-life offspring production in response to early-life reproductive investment.
Female Lifespan Is Reduced by Early-Life Mating with Vasectomized Males.
Experiments in rodents have repeatedly documented a cost to lifespan when reproduction is permitted across life, observed in a variety of laboratory (15) and wild-derived (16) strains. This represents a more widespread trade-off observed across a variety of animal species in the laboratory and in the wild (17). By comparing females housed with males of different gonadal status over the first 300 d of life, then housed with another female, we were able to test whether male presence contributes to the lifespan effects previously documented with reproduction in mammals. Females housed with different partners over the first 300 d of life had significantly different lifespans (log-rank test, P = 0.024) (Fig. 3). This was a consequence of the shorter lifespan in females housed with vasectomized females compared with females housed with other females (P = 0.014). This indicates that mating without fertilization has a deleterious effect on survival of female mice. The effect of pairing with an intact male over the first 300 d was not associated with a significant reduction in lifespan compared with females housed with other females (P = 0.14), although some animals were censored from the lifespan analysis due to birthing difficulties in the first 300 d. The median and maximal lifespan values for each cohort of animals are presented in SI Appendix, Table S3, and individual dates of birth and death are provided in Dataset S1.
Housing with a vasectomized male early in life reduces female lifespan. Survival curves for females housed in different social environments before 300 d and then housed with another female. Each dot represents a time point at which an individual mouse died. The red asterisk represents the difference in survival between those previously housed with females and those previously housed with vasectomized males (P < 0.05), calculated with a log-rank test.
Persistent Alterations in Circulating Prolactin Are Associated with Life History Responses to Mating.
The life history consequences of vasectomized male presence could be elicited by various different processes occurring during social interactions, including male contact, transfer of seminal fluid, and response to perception of male cues. In rodents, male stimuli perceived during mating causes pseudopregnancy, in which coital stimulation generates a neuroendocrine response analogous to early pregnancy (6), driven by diurnal surges in prolactin secretion (18). It has been shown that prolactin surges from actual pregnancy and lactation can lead to reduced prolactin secretion in reproductively experienced animals, which is associated with enhanced future neuronal sensitivity to this hormone and improved expression of maternal behavior (19). Since pseudopregnancy also results in prolactin exposure, we tested whether altered prolactin secretion occurs after mating experience with vasectomized males and could be linked to changes in life history.
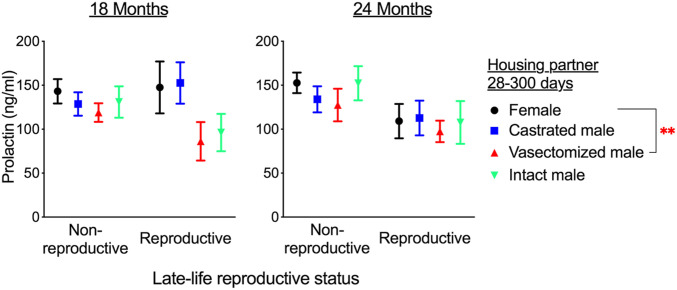
We assessed prolactin concentration in plasma samples collected from mice at age 18 and 24 mo, including samples both from mice permitted to reproduce late in life (Fig. 2) and those housed in nonreproductive environments (Fig. 3). Analyzing data from both time points showed a significant effect of early-life social environment (e.g., pre-300 d of age) on prolactin level (effect of early-life treatment: F3, 157.58 = 2.69, P = 0.049). Females housed with vasectomized males from age 21 d to 300 d had lower concentrations of plasma prolactin at 18 and 24 mo compared with females housed with other females during early life (Fig. 4). In addition, there was an effect of late-life treatment (e.g., allowed to reproduce or not; F1, 169.23 = 10.09, P = 0.002), and no interaction between these two factors (interaction between early and late-life treatments: F4, 256.96 = 1.62, P = 0.28). This demonstrates that those females housed with males later in life also show a reduction in circulating prolactin (Fig. 4), and this effect is additive to the effects of early-life social conditions. Therefore, male interactions can lead to life-long changes in circulating prolactin that persist in different social environments and correlate with altered life history and aging.
Persistent suppression of circulating prolactin after mating. Data are split to show the effect of both early-life conditions (colored bars) and housing in a nonreproductive (with a female) or reproductive (with male) environment when samples were obtained. Data represent mean ± SEM. **P < 0.01 for contrast between females previously housed with vasectomized males and those housed previously with other females (red asterisk), calculated from a generalized linear mixed model including data from both the 18 mo and 24 mo sampling points and both groups of late-life reproductive status. n = 13 to 25 per group at 18 mo and 11 to 25 per group at 24 mo.
Discussion
Our results show that the presence of a male alone dramatically affects female life history in mice, promoting increases in body weight that can persist across life, enhancing reproductive output when later mating with fertile males but leading to reductions in lifespan. This indicates that costs and consequences of reproduction typically attributed to pregnancy and lactation in mammals (3, 4) may also be a consequence of interactions that occur due to a male's presence, such as mating, irrespective of investment in parental care.
Our study does not identify which aspects of male presence induce these changes. Females paired with castrated males showed no change in life history or aging. Thus, we can exclude the possibility that the effects arise as a consequence of differences in the number of females to which individuals are exposed, and infer that the effects are linked to male traits expressed under androgen-dependent control, such as mating and some olfactory sexual signals.
The effects of mating on female lifespan in invertebrates are often attributed to consequences of seminal fluid transfer, with seminal fluid proteins released in male ejaculate evolving with intensity of sexual conflict (20), promoting female egg-laying after the current mating (21) but reducing female lifespan (8). There is evidence that seminal fluid proteins have diversified over rodent evolution, with specific proteins evolving in line with the predicted intensity of sperm competition (22), and thus also with sexual conflict. Exposure to seminal fluid in mammals also facilitates implantation via induced inflammatory responses, ultimately promoting fetal growth (23). Whether inflammatory responses to seminal fluid have systemic effects on maternal physiology and, ultimately, subsequent life history consequences for females present a fruitful avenue for future study.
Female rodents show adaptive self-regulated neuroendocrine responses to mating that sustain reproductive hormone levels during the first half of pregnancy. This is exemplified by the occurrence of pseudopregnancy, in which sterile mating leads to neuroendocrine hormonal responses that mimic early pregnancy (6). Pseudopregnancy is a robust response that occurs in ∼80% of matings with vasectomized male mice (24). Thus, females in our vasectomized male treatment cycled through repeated pseudopregnancies. In contrast, castrated males did not induce pseudopregnancy, because they do not mate. Therefore, from 28 to 300 d of age, females paired with vasectomized males would have been exposed to an increased frequency of pseudopregnancies compared with other treatment groups, and the hormone responses occurring during these periods could have long-lasting effects influencing life history.
Pseudopregnancy and the first half of actual pregnancy are sustained by diurnal surges of prolactin that occur for ∼12 d after mating (25). Such hormonal exposure can lead to neuroendocrine adaptations that help promote reproduction in subsequent events. Late-life circulating prolactin levels were lower in females that had been earlier paired with vasectomized males compared with controls, an effect consistent in both late-life social environments. A reduction in circulating prolactin can indicate increased hormone sensitivity from enhanced hypothalamic feedback (26). In relation to previous parity, these neuroendocrine adaptations have been shown to enhance maternal behaviors, such as pup retrieval (19). If the decreased circulating prolactin levels in females previously paired with vasectomized males reflects enhanced hypothalamic feedback, this could contribute to those females’ improved late-life fecundity.
Prolactin secretion is regulated by additional hormonal axes, including stress responses (27) and growth hormone signaling (28). Furthermore, the activities of these additional hormone systems can themselves be altered by social conditions (29). Thus, initial changes in other interconnected hormone systems, such as diminished stress responses with mating (29), might also lead to the observed relationship between social conditions and circulating prolactin. Irrespective of the initial trigger, changes in reproductive, growth, and/or stress hormone axes are good candidates for signals that could lead to life history alterations in response to social cues. In Caenorhabditis elegans, specific insulin signals are activated and influence lifespan in response to male pheromone exposure, while mechanistic target of rapamycin signaling contributes to hastened aging in response to seminal fluid (11, 30). Thus, several different signaling pathways could be activated by mating cues, each potentially leading to life history and aging responses. Our study does not determine the mechanism underlying changes in female lifespan after exposure to vasectomized males early in life, but the response is sufficient to reduce late-life female survival after males have been removed. Further research is needed to determine whether changes in survival are associated with specific pathways or diseases that lead to death, and whether this is associated with declines in late-life physical function or other aspects of aging.
The lifespan reduction from mating is significant, although relatively modest, occurring as a consequence of environmental conditions experienced over the first third of life. It would be of interest to determine whether these effects are stronger in females mated, without fertilization, across their whole life. Indeed, in contrast to previous studies showing a reduced lifespan as a consequence of repeated pregnancies across the entire life (15, 16), we saw no significant change in the lifespan of females mated with intact males over the first 300 d only, when compared with females without male exposure. It is possible that the effects of mating, pregnancy, and lactation are cumulative and increase with the duration of exposure.
Females with intact males will not mate while pregnant (21 d), whereas females paired with vasectomized males return to estrous after an ∼12-d pseudopregnancy. Thus, the higher mating rate in females paired with vasectomized males could have influenced their lifespan. Such an effect of mating rate has been reported in Drosophila melanogaster (31). Vasectomized and intact males might also differ in their social interactions with females, with intact males exposed to pups in their cage. We observed no differences in aggression or female injury between those housed with intact males and those housed with vasectomized males, and we have previously observed that housing females with vasectomized males actually reduces corticosterone levels in cohabiting females (32). However, a detailed study of social interactions, combined with manipulations of the frequency of mating exposure and seminal fluid transfer, is needed to resolve the underlying male factor generating these responses.
There is an increasing appreciation that life history can be modulated by social interactions and even perceived cues of the social environment (29, 33). While experimental evidence and underlying mechanisms have been recently probed in invertebrates (9, 11), it is well established that mammals can modulate sexual maturity, estrous cycling, and maintenance of pregnancy in response to conspecific scent cues (5, 34). Our results suggest that sexual cues and social interactions experienced during specific periods of life can have effects that persist across the entire lifespan of some female mammals. In humans, women can show long-lasting changes in adiposity and prolactin secretion with parity, changes that remain detectable for at least a decade after childbirth (35–38). Understanding the mechanisms that underlie these effects, and the specific cues and reproductive processes that drive these responses, could help reveal how life course factors (e.g., childrearing, sexual cohabitation) lead to sex-based differences in physiology and sex-specific health disparities.
Methods
Animals.
This research was approved by the University of New South Wales (UNSW) Animal Care and Ethics Committee (approval 15/70B). All experiments were carried out in accordance with approved guidelines. Mice were the C57BL/6J strain, in which the consequences of castration and vasectomy on male mating behavior and androgen-dependent sexual signaling are well established, with castration known to rapidly reduce male mating behavior (13, 39). Mice were purchased from the Australian BioResource Center (stock C57BL/6JAusb), which are maintained as a stock of C57BL/6J mice with imports of the same strain of animals from The Jackson Laboratory.
Experiments were run in four equal-sized batches, with treatment groups spread evenly across batches. Female mice were shipped to UNSW at 3 wk of age. For male mice, before being shipped to UNSW, animals were castrated, vasectomized, or sham vasectomized (intact) at age 6 to 8 wk. For all surgeries, animals were anesthetized with a ketamine and medetomidine mix, then administered ketoprofen as analgesia. An incision in the abdomen was made and, in vasectomized males, both vas deferens were cauterized. In castrated males, both testes where also removed during the procedure. Intact (sham) males underwent the same surgical incision with the vasa deferentia revealed but not cauterized.
The mice were allowed 1 wk to recover, then sent to UNSW, where they were allowed another 1 wk to habituate before being housed in different social conditions. The mice were maintained at 22 ± 2 °C on a 12:12-h reverse light/dark cycle, with the dark period starting at 9 AM. They were fed Mouse and Rat Maintenance Pellets (Gordon’s Specialty Stock Feeds). The light cycle was reversed so that experimental manipulations occurred in the dark period under dim red light.
Experimental Procedures.
All experimental females were housed with a female sibling; then at age 28 d, these sibling pairs were allocated at random to housing with either an additional unfamiliar female, a castrated male, a vasectomized male, or an intact male (n = 54 per group, 3 animals per cage). Housing partners were age 9 to 10 wk at pairing. For females paired with intact males, offspring remained within the home cage until they were weaned and removed from the cage at age 21 d. All females produced litters over this time period (average number of litters produced, 8.77; range, 2 to 12). At 300 d of age (Fig. 1A), original housing partners were removed, and females subsequently remained with their female sibling (n = 30 per early life social group, 2 animals per cage) or with their female sibling and a 9- to 10-wk-old intact male (n = 24 per early life social group, 3 animals per cage). Females were housed in open-top cages (48 × 11.5 × 12 cm) in the same room, with the cages of females in different groups assorted at random across racks. Thus, females in different treatment groups were exposed to equivalent levels of background auditory and olfactory cues emanating from outside the cage in which they were housed. All females were weighed at age 2, 3, 6, 12, 18, and 24 mo, and at 6, 18, and 24 mo, a small blood sample was collected during the first 3 h of the dark period in nonfasted animals. After a brief period under a heat lamp, a small incision was made at the base of the tail. A blood sample of no more than 100 μL was collected into a EDTA-coated tube, after which plasma was obtained and stored at −80 °C until use in analysis.
Assessment of Late-Life Reproduction.
Females were allowed to breed continuously with intact males after these individuals were placed with females at age 300 d. Offspring remained within the home cage until they were weaned and removed at age 21 d. At this time point, the number and weight of offspring was recorded. After the switch to housing with intact males, several females died as a consequence of birthing difficulties during their first litter. These animals were removed from the analysis (3 out of 24 mice in females previously paired with females or castrated males; 4 in females those previously housed with vasectomized males, and 1 in those previously housed with intact males). To assess fecundity, we recorded the number of offspring weaned (at 21 d) by each female, litter weight, number of litters produced, interlitter interval, and age at last litter. To ensure that we assessed total fecundity from 300 d, females were monitored until death, although the last litter recorded was in a 610-d-old female.
Assessment of Lifespan.
As is typical in mouse lifespan studies (40), females were housed just with their siblings after 300 d for assessment of lifespan. Females remained in these standard conditions until death or until they were considered to be so severely moribund that they were unlikely to survival an additional 48 h (40). Severe moribundity was indicated by one or more of the following clinical signs: rapid weight loss, unresponsiveness to manual stimulation, trembling/hunched or immobile posture, labored and irregular breathing, severe abdominal enlargement (unrelated to pregnancy), or a severely ulcerated tumor. The age at which a moribund mouse was euthanized served as an estimate of its lifespan, and mice found dead were also noted at each daily inspection. Several females housed with intact males early in life were removed from the population because of severe fighting with males or birthing difficulties. For survival analysis, these animals were censored.
Prolactin Assay.
For all available samples from animals at age 18 and 24 mo, prolactin was assessed using an enzyme-linked immunosorbent assay with antibodies and standards from the US National Hormone and Peptide Program, as described previously (41). A pooled mouse plasma sample run across plates in duplicate showed an intraplate assay coefficient of variability between 0.9% and 4.7% (i.e., variation in control sample value within plates) and an the interplate assay coefficient of variability of 2.28% (i.e., variation in control sample value across plates).
Statistics.
Statistical analyses were carried out in SPSS version 25 (IBM). Before analysis, data were visually assessed to determine conformation to parametric assumptions of specific tests, and when necessary, data were log-transformed to fit with the associated assumptions. Following our previous study (32), we first tested whether there was an overall significant effect of early-life social environment across the four treatment groups on a trait of interest. When this treatment effect was significant (P < 0.05), we conducted planned contrasts to test which of the male housing environments differed from that of controls (housed with other females).
We used a repeated-measures generalized linear model to assess changes in body weight, analyzing pre- and post-300 d body weight data separately as females were housed in different environments. For assessment of fecundity, we used GLMMs, including both Dam ID and sibling pair as random effects to control for repeated litters produced by the same individual and the possibility of communal nursing in females housed together in a cage. Previous social treatment and time since pairing were included as fixed effects. For assessment of prolactin at age 18 and 24 mo, we used a GLMM, including Dam ID as a random effect to control for samples collected from the same female at different time points, early- and late-life treatments as separate fixed effects, and animal age/plate as an additional fixed effect (samples from different ages were run on several different plates). Survival data were analyzed using the log-rank test.
Data Availability.
All pertinent data are available on the Open Science Network (https://osf.io/y49p2/).
Supplementary Material
Supplementary File
Supplementary File
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
All pertinent data are available on the Open Science Network (https://osf.io/y49p2/).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003159117/-/DCSupplemental.