- Journal List
- FASEB J
- PMC5998970

Serum vitamin C levels modulate the lifespan and endoplasmic reticulum stress response pathways in mice synthesizing a nonfunctional mutant WRN protein
Lucie Aumailley
*Centre de Recherche du Centre Hospitalier de l’Université (CHU) de Québec, Faculté de Médecine, Université Laval, Quebec City, Quebec, Canada;
Marie Julie Dubois
†Institut Universitaire de Cardiologie et de Pneumologie de Québec, Faculté de Médecine, Université Laval, Quebec City, Quebec, Canada;
Tracy A. Brennan
‡Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA;
Chantal Garand
*Centre de Recherche du Centre Hospitalier de l’Université (CHU) de Québec, Faculté de Médecine, Université Laval, Quebec City, Quebec, Canada;
Eric R. Paquet
§Centre de Recherche sur le Cancer de l’Université Laval, Hôpital Hôtel-Dieu de Québec, Quebec City, Quebec, Canada
Robert J. Pignolo
¶Department of Medicine, Mayo Clinic College of Medicine, Rochester, Minnesota, USA
André Marette
†Institut Universitaire de Cardiologie et de Pneumologie de Québec, Faculté de Médecine, Université Laval, Quebec City, Quebec, Canada;
Michel Lebel
*Centre de Recherche du Centre Hospitalier de l’Université (CHU) de Québec, Faculté de Médecine, Université Laval, Quebec City, Quebec, Canada;
Associated Data
- Supplementary Materials
- fj.201701176R.sf1.jpg (914K)GUID: 39CA64EB-D22F-4C6A-9EC2-03AE88C4C4ECfj.201701176R.sf2.jpg (1.5M)GUID: 7828EAB0-9771-43FF-99D5-DA2DFE9DEC4Cfj.201701176R.sf3.jpg (865K)GUID: 40770B0C-AE37-417C-BF0A-90586EDDEF76fj.201701176R.sf4.jpg (1.0M)GUID: A864F0C1-9A2A-4816-BD80-625F98E2836Afj.201701176R.sf5.jpg (426K)GUID: 00554C09-BAF4-47A8-A971-E606B26DCE1Efj.201701176R.st1.xlsx (193K)GUID: 1B549616-FFE7-4E74-91FF-464F68A97C5Afj.201701176R.st2.xlsx (42K)GUID: 830D5EC6-0E9C-4438-9F38-9C0EAD9D7722fj.201701176R.sd1.xlsx (62K)GUID: 7A5BCC95-F078-498E-BEE2-88A024A96537
Abstract
Werner syndrome (WS) is a premature aging disorder caused by mutations in a RecQ-family DNA helicase (WRN). Mice lacking part of the helicase domain of the WRN ortholog exhibit several phenotypic features of WS. In this study, we generated a Wrn mutant line that, like humans, relies entirely on dietary sources of vitamin C (ascorbate) to survive, by crossing them to mice that lack the gulonolactone oxidase enzyme required for ascorbate synthesis. In the presence of 0.01% ascorbate (w/v) in drinking water, double-mutant mice exhibited a severe reduction in lifespan, small size, sterility, osteopenia, and metabolic profiles different from wild-type (WT) mice. Although increasing the dose of ascorbate to 0.4% improved dramatically the phenotypes of double-mutant mice, the metabolic and cytokine profiles were different from age-matched WT mice. Finally, double-mutant mice treated with 0.01% ascorbate revealed a permanent activation of all the 3 branches of the ER stress response pathways due to a severe chronic oxidative stress in the ER compartment. In addition, markers associated with the ubiquitin-proteasome–dependent ER-associated degradation pathway were increased. Augmenting the dose of ascorbate reversed the activation of this pathway to WT levels rendering this pathway a potential therapeutic target in WS.—Aumailley, L., Dubois, M. J., Brennan, T. A., Garand, C., Paquet, E. R., Pignolo, R. J., Marette, A., Lebel, M. Serum vitamin C levels modulate the lifespan and endoplasmic reticulum stress response pathways in mice synthesizing a nonfunctional mutant WRN protein.
Werner syndrome (WS) is a human autosomal recessive disorder characterized by genomic instability and the premature onset of several age-related diseases (1–4). The defective enzyme responsible for WS is a DNA exonuclease/helicase involved in DNA repair, replication, transcription, and telomere maintenance (5–9). Several years ago, we generated a mouse model with a deletion in the helicase domain of the murine WS RecQ-like helicase gene (Wrn) ortholog (10) (referred to as Wrn∆hel/∆hel, hereafter). This deletion abolishes both the helicase and exonuclease activities of the mouse Wrn protein (11). Wrn∆hel/∆hel mice develop abnormally high visceral fat content, hepatic steatosis (12), and aortic stenosis followed by cardiac fibrosis (13). In addition, Wrn∆hel/∆hel mice show an increase in genomic instability with age, an increase in the incidence of several cancer types, and a reduced mean lifespan of ∼17% compared with wild-type (WT) mice (14, 15). Similar to patients with WS (16), the antioxidant status (levels of vitamin C or ascorbate and glutathione) in 9-mo-old Wrn∆hel/∆hel mice is abnormally high in blood compared with age-matched WT mice (15). This difference suggests that Wrn∆hel/∆hel mice are trying to cope with an unbalanced cellular redox status or that the Wrn protein affects genes involved in the oxidative stress response (17). Accordingly, we found that untreated Wrn∆hel/∆hel mouse embryonic fibroblast redox status is similar to WT mouse embryonic fibroblasts that have been treated with hydrogen peroxide (18). We tested the impact of 3 known antioxidants (catechin, resveratrol, and ascorbate) on the health of Wrn∆hel/∆hel mice (12, 15, 19). Surprisingly, the most efficient antioxidant in reversing the observed phenotypes in Wrn∆hel/∆hel mice was ascorbate, despite the fact that mice, unlike humans, synthesize their own ascorbate (15). In this study, we generated Wrn∆hel/∆hel mice that, like humans, rely entirely on diet to obtain ascorbate, to better assess the impact of ascorbate on this mouse model. The murine enzyme (absent in humans) required for de novo ascorbate synthesis is the gulonolactone oxidase encoded by the Gulo gene. Without ascorbate supplementation in their diet, Gulo null (Gulo−/−) mice become rapidly sick, similar to humans (20). We examined the effect of 0.01 and 0.4% (w/v) ascorbate supplementation in drinking water on WrnΔhel/Δhel/Gulo−/− mice. A 0.4% ascorbate supplementation has been shown to significantly extend the lifespan of Wrn∆hel/∆hel mice (21).
MATERIALS AND METHODS
Mice
Gulo−/− mice were obtained from the Mutant Mouse Regional Resource Centers (University of California–Davis, Davis, CA, USA) and were housed at the Centre de Recherche en Cancérologie de l’Université Laval animal facility. Mice lacking part of the helicase domain of the Wrn gene were generated by homologous recombination (10). All mice were backcrossed onto C57BL/6 background for 12 generations. Mice of all required genotypes were obtained by mating homozygous WrnΔhel/Δhel individuals with Gulo−/− mice and intercrossing the F1 and F2 generations. Care of mice was in accordance with the guidelines of Laval University’s Animal Protection Committee (Permit 2014029). Mice were housed in cages (containing a top filter) on static racks in a conventional animal facility at 22 ± 2°C with 40–50% humidity and a 12-h light–dark cycle (light cycle: 06:00–18:00 h). All mice were fed ad libitum with the standard 2018 Teklad Global 18% Protein Rodent Diet (Envigo, Madison, WI, USA). Mice were checked every day, and those that became immobile or moribund were euthanized for histologic examination of their organs (14). Euthanasia was performed with 3% isoflurane (general anesthesia) followed by cervical dislocation. One cohort of Gulo−/− mice and 1 cohort of WrnΔhel/Δhel/Gulo−/− mice were maintained on a standard diet and supplemented with 0.01% of l-ascorbate (w/v) in drinking water from weaning until the ages indicated in the analyses. A second cohort of WrnΔhel/Δhel/Gulo−/− mice was given 0.4% l-ascorbate (w/v) in drinking water for the various times indicated. WT control C57BL/6 mice were maintained in the same room from weaning until the age of 4 or 20 mo with normal drinking water (no ascorbate supplement). Finally, WrnΔhel/Δhel mutant mice were maintained under standard diet with no ascorbate supplementation.
Histologic examination
Tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Thin sections were mounted on glass slides and stained with hematoxylin and eosin (H&E). TUNEL assays for detection of apoptotic cells were performed on 4–5 µm paraffin-embedded sections with an In situ Apoptosis Detection Kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s recommendations.
Metabolite measurements
Blood was drawn at 10 am by cardiac puncture and exsanguination under anesthesia at the age of 4 mo and processed (21). Ascorbic acid in serum was measured with the ferric reducing ascorbate assay kit from BioVision Research Products (Mountain View, CA, USA). Metabolite measurements were performed by Biocrates Life Sciences metabolomic services (Biocrates Life Sciences, Innsbruck, Austria). In brief, Biocrates’ commercially available kit plates were used for quantification of amino acids, acylcarnitines, sphingomyelins, phosphatidylcholines, hexoses, and biogenic amines. The fully automated assay was based on phenylisothiocyanate derivatization in the presence of internal standards followed by flow injection analysis–tandem mass spectrometry (MS) (acylcarnitines, lipids, and hexose) and liquid chromatography (LC)/MS (amino acids, biogenic amines) with a 4000 QTrap mass spectrometer (AB Sciex, Darmstadt, Germany) with electrospray ionization. The experimental metabolomics measurement technique has been described in Illig et al. (22). Eicosanoids and other oxidized polyunsaturated fatty acids were extracted from samples with aqueous acetonitrile that contained deuterated internal standards. The metabolites were determined by LC-MS/MS with multiple-reaction monitoring in negative mode on a 4000 QTrap mass spectrometer with electrospray ionization. The LC-MS/MS method used for the analytical determination of eicosanoids has been published (23). Accuracy of the measurements was in the normal range of the method (deviations from target, ≤20%) for all analytes. In total, 203 different metabolites were measured. The metabolomics data set contains 21 aa, 19 biogenic amines, 1 hexose (H1), free carnitine (C0), 40 acylcarnitines (Cx:y), hydroxylacylcarnitines [C(OH)x:y], and dicarboxylacylcarnitines (Cx:y-DC), 15 sphingomyelins (SMx:y), N-hydroxylacyloylsphingosylphosphocholine [SM (OH)x:y], 77 phosphatidylcholines (PC), 14 lyso-phosphatidylcholines, and 17 eicosanoid acids and prostaglandins. Lipid side-chain composition is abbreviated as Cx:y, where x denotes the number of carbons in the side chain and y the number of double bonds. For example, PC acyl-alkyl (PC ea) C30:1 denotes an acyl-alkyl phosphatidylcholine with 30 carbons in the 2 fatty acid side chains and 1 double bond in one of them (22). The precise position of the double bonds and the distribution of the carbon atoms in different fatty acid side chains cannot be determined with this technology. Full biochemical names and raw data are provided in the Excel spreadsheets of Supplemental Data S1.
Cytokine measurements
The following cytokines were assessed in the serum (diluted 1:4) using the multiplex kit from Bio-Rad Laboratories (MD000000EL; Mississauga, ON, Canada): IL-15, IL-18, leukemia inhibitory factor, macrophage–colony-stimulating factor (M-CSF), monokine induced by γ-interferon (MIG), macrophage inflammatory protein (MIP)-2, platelet-derived growth factor-BB, VEGF, and fibroblast growth factor (FGF) basic. The following cytokines were assessed in the serum (diluted 1:4) using the Multiplex Kit from Bio-Rad Laboratories (MD60009RDPD): IL-1α, -1β, -2, -3, -4, -5, -6, -10, -12 (p40), -12 (p70), -13, and -17; katacalcin (KC); monocyte chemoattractant protein-1; MIP-1α and -1β; TNF-α; regulated on activation, normal T-cell–expressed and –secreted (RANTES); Eotaxin; granulocyte–colony-stimulating factor (G-CSF); granulocyte-macrophage–colony-stimulating factor (GM-CSF); and IFN-γ. The metabolic hormones C-peptide 2, gastric inhibitory polypeptide, leptin, and resistin were assessed in serum with the Milliplex Mouse Metabolic Magnetic Bead Panel (MMHMAG-44K) from MilliporeSigma (Billerica, MA, USA). The following cardiovascular risk factors were assessed in the serum (diluted 1:20) using the Milliplex Mouse CVD Panel 1 Kit from MilliporeSigma (MCVD1MAG-77K): soluble E- and P-selectin, intercellular adhesion molecule-1, platelet endothelial cell adhesion molecule-1, pro-matrix metalloproteinase (MMP)-9, thrombomodulin, and total plasminogen activation inhibitor (PAI)-1. Full biologic names and raw data are provided in the Supplemental Table S1. All measurements were performed on a Bio-Plex 200 System (Bio-Plex Manager Software v.6.0; Bio-Rad Laboratories.)
Microcomputerized tomography analysis of trabecular and cortical bone
High-resolution images of femurs were acquired by using a Scanco VivaCT 40 (Bruettisellen, Switzerland). The femurs were scanned with the source voltage of 55 kV, a source current of 142 µA, and an isotropic voxel size of 10.5 µm. After the scanning, 3D microstructural image data were reconstructed and structural indices were calculated with Scanco µCT V6.1 Software. The number of femurs from 4-mo-old mice analyzed by micro-CT analysis were n = 8 WT, n = 10 WrnΔhel/Δhel, n = 10 Gulo−/−, n = 12 WrnΔhel/Δhel/Gulo−/− mice with supplemental 0.01% ascorbate, and n = 8 WrnΔhel/Δhel/Gulo−/− mice treated with supplemental 0.4% ascorbate.
The area for the trabecular analysis started 57.5 µm proximal from the growth plate at the distal end of the femur and extended proximally 1050 µm toward the femoral head. The region of interest (ROI) for trabecular microarchitectural variables was defined by manually outlining the bone within the endocortical margins, allowing a few voxels between the ROI and the endocortical margin (24). An upper threshold of 1000 Hounsfield units and a lower threshold of 220 Hounsfield units were used to delineate each pixel as bone or nonbone. Representative 2-dimensional cross-sectional images for each genotype within the young and aged groups are shown in gray scale. Trabecular bone volume per total volume (%), mean trabecular thickness (mm), mean trabecular number (1/mm), and mean trabecular separation (mm) indices were computed.
Fractionation procedures for tissues
Total liver protein extracts were prepared as described elsewhere (21). Total endoplasmic reticulum (ER) fractions from mouse livers were obtained with an ER Enrichment Assay Kit (Novus Biologicals, Burlington, ON, Canada) according to the manufacturer’s protocol. Enriched ER pellets were resuspended in suspension buffer containing phosphatase inhibitor cocktail (PhosStop Easy Pack) from Roche Applied Sciences (Indianapolis, IN, USA).
Western blot analyses
Western blot analyses were performed as described in Aumailley et al. (21). When indicated, immunoblots were probed with the following antibodies: rabbit polyclonal antibodies raised against the eukaryotic translation initiation factor 2-α kinase 3 [anti-PERK (H300): sc-13073]; eukaryotic translation factor 2-α [anti-eIF2α (FL-315): sc-11386]; phosphorylated eukaryotic translation factor 2-α [anti-p-eIF2α (Ser 52): sc-101670]; heat shock cognate (HSC) 70 kDa protein [anti-HSP70/HSC70 (H300): sc-33575]; cAMP-dependent transcription factor (ATF)-4 [anti-CREB-2 (C-20): sc-200]; ubiquitin [anti-Ub (FL-76): sc-9133]; homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member-1 [anti-HERP (H-59): sc-98669]; and Werner syndrome protein [anti-WRN (H-300): sc-5629] were from Santa Cruz Biotechnology (Dallas, TX, USA). A mouse mAb against ATF-6α [anti-ATF-6α (F-7): sc-166659] and C/EBP-homologous protein (CHOP) [anti-GADD153 (B-3): sc-7351] was from Santa Cruz Biotechnology; rabbit mAb against inositol-requiring kinase-1α [anti-inositol-requiring enzyme (IRE)-1 (14C10) 3294], protein disulfide isomerase [anti-protein disulfide isomerase (PDI) (C81H6) 3501] from Cell Signaling Technology (Beverly, MA, USA); a rabbit pAb against glucose-related protein-78 (anti-GRP78) from Proteintech (Chicago, IL, USA); a rabbit pAb against calreticulin (anti-calreticulin; ER marker ab39818) from Abcam (Cambridge, United Kingdom); and a mouse mAb against actin (A5441) from MilliporeSigma.
Reactive oxygen species measurements in liver tissues and ER fractions from mouse livers
Liver lysates in RIPA buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM PMSF, and complete protease inhibitor cocktail (Roche Applied Science)] or ER enriched fractions from mouse livers were incubated with 10 µg/ml of the dye 2′-7′ dichlorofluorescein diacetate (DCFA) (MilliporeSigma) for 1 h at 37°C. As controls, RIPA buffer or ER suspension buffer was also incubated with DCFA, and 100 µl (500 µg of liver proteins or 100 µg of ER fractions) of the samples was put into 96-well plates. Fluorescence was measured with a SpectraMax i3x Multi-Mode microplate reader (Molecular Devices, Sunnyvale, CA, USA). The excitation and emission wavelengths used were 485 and 527 nm, respectively.
Protein sulfhydryl measurements in total liver tissues
Weighed liver tissues were homogenized on ice in 20 mM sodium phosphate buffer (pH 7.4) containing 140 mM potassium chloride. Samples were then incubated in lysis buffer for 30 min on a rotary device at 4°C. Protein sulfhydryls were measured by monitoring the reaction between free thiol groups on proteins (240 µg of liver proteins) and the colorimetric reagent 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB; MilliporeSigma) (25, 26).
Statistical analysis
We used a 1-sided Fisher exact test for comparison between the mitochondrial mutation rates between all genotypes. A Student’s t test was performed when indicated between different cohorts of mice. Data in graphs are presented ± sem, and differences between groups were considered significant when P < 0.05. The identification of significantly different metabolites and cytokines was performed using the Kruskal-Wallis nonparametric test. Principal component analysis (PCA) and presentation of the results using heat maps were performed using R v.2.14 (https://www.r-project.org/). We considered events significant at P < 0.01. Heat maps were generated using Euclidian distance and complete agglomerative methods. One-way ANOVA followed by Tukey’s honest significant difference test or Sidak’s multiple-comparisons test for post-ANOVA pair-wise comparisons was performed for the morphologic studies and for the Western blot analyses. Differences were considered significant when P < 0.05.
RESULTS
Lifespan and body weight of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01 and 0.4% ascorbate
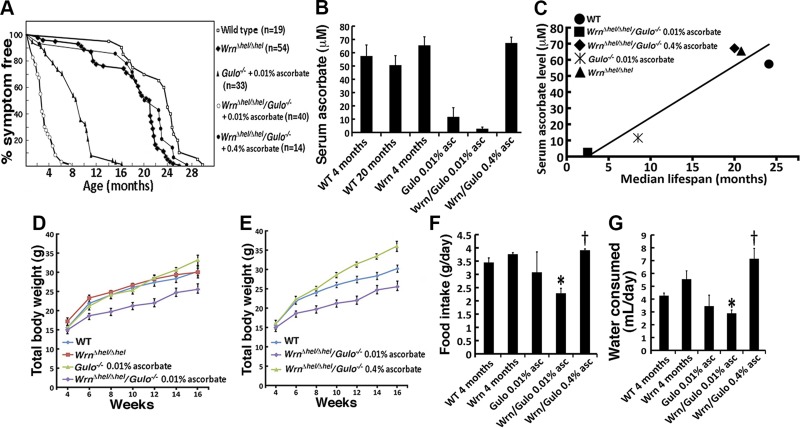
Gulo−/− and Wrn∆hel/∆hel mice were crossed to generate Wrn∆hel/∆hel/Gulo−/− mice to assess the role of ascorbate (vitamin C) on the lifespan of double-mutant homozygous mice. Littermates of all genotypes (males and females) were carefully followed throughout the years and scored for the occurrence of any sign of disease. Since WT and Wrn∆hel/∆hel mice synthesize their own ascorbate, they did not receive supplemental ascorbate in this study. The maximum lifespan of Wrn∆hel/∆hel and WT mice was 26 and 30 mo of age, respectively (Fig. 1A). As Gulo−/− mice become rapidly ill without ascorbate, 0.01% of sodium ascorbate (w/v) was added to drinking water at weaning. Under these conditions, 50% of the Gulo−/− mice survived for at least the first 8 mo without any problem and were fertile. The oldest Gulo−/− animal died at the age of 16 mo. The Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate were smaller and sterile, became sick, and had to be humanely euthanized once they had lost more than 20% of their total body weight. The median lifespan of the Wrn∆hel/∆hel/Gulo−/− mice was ∼3 mo. The oldest Wrn∆hel/∆hel/Gulo−/− animal died at the age of ∼8 mo. The difference in lifespan between Wrn∆hel/∆hel/Gulo−/− and Gulo−/− mice treated with 0.01% ascorbate was significant (log rank test; P = 3.2 × 10−12). When we supplemented drinking water with 0.4% ascorbate, 50% of the Wrn∆hel/∆hel/Gulo−/− mice survived for at least the first 20 mo without any problem. The oldest Wrn∆hel/∆hel/Gulo−/− mouse treated with 0.4% ascorbate was euthanized at the age of 27 mo as it became moribund. These mice exhibited splenomegaly, enlarged mesenteric lymph nodes, myeloid leukemias, or liver tumors. The difference in lifespan between Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 and 0.4% ascorbate was significant (log rank test; P = 4.4 × 10−9). The lifespan of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate was not significantly different compared with WT mice (log rank test; P = 0.137). However, the lifespan of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate was also not significant to untreated Wrn∆hel/∆hel mice (log rank test; P = 0.134).
Effect of ascorbate on growth and lifespan of WrnΔhel/Δhel/Gulo−/− mice. A) Percentage of disease-free animals according to age for WT, WrnΔhel/Δhel, Gulo−/−, and WrnΔhel/Δhel/Gulo−/− mice. B) Serum ascorbate levels in different groups of our mouse cohort (n = 6 per group). Bars represent sem. Groups of mice are labeled in the graph: WT, wild type; Wrn, WrnΔhel/Δhel mice; Gulo 0.01% asc: Gulo−/− mice given 0.01% supplemental ascorbate since weaning; Wrn/Gulo 0.01% asc, WrnΔhel/Δhel/Gulo−/− mice given 0.01% ascorbate since weaning; and Wrn/Gulo 0.4% asc, WrnΔhel/Δhel/Gulo−/− mice given 0.4% ascorbate since weaning. C) Correlation of serum ascorbate level and median lifespan. Graph showing the correlation between serum ascorbate level and median lifespan (Pearson’s correlation coefficient r = 0.942; P = 0.017). D) Total body weight of the different groups of mice (n = 10) from the age of 4–16 wk. Bars represent sem. E) Comparison of total body weight (age 4–16 wk) of untreated WT and WrnΔhel/Δhel/Gulo−/− mice treated with either 0.01 or 0.4% ascorbate (n = 10). Bars represent sem. F) Food intake of mice (n = 3–6) at 4 mo of age. Bars represent sem. Groups of mice are labeled as in panel B. *P < 0.044 vs. WT mice; †P < 0.011 vs. WrnΔhel/Δhel/Gulo−/− mice given 0.01% supplemental ascorbate (by Tukey test). G) Water consumption of mice (n = 3–6) at 4 mo of age. Bars represent sem. *P < 0.044 vs. WT mice; †P < 0.011 vs. WrnΔhel/Δhel/Gulo−/− mice given 0.01% ascorbate (by Tukey test).
We next measured the amount of serum ascorbate in mice at 4 mo of age. The analysis also included 20-mo-old untreated WT mice. Four-mo-old Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% of ascorbate since weaning had a significant decrease in serum ascorbate compared with untreated WT and Wrn∆hel/∆hel mice (Fig. 1B). Supplemental 0.4% ascorbate in the drinking water increased serum ascorbate level in the Wrn∆hel/∆hel/Gulo−/− mice to WT and Wrn∆hel/∆hel levels (Fig. 1B). ANOVA between groups of mice indicated a P < 0.01. The P values from the post-ANOVA Tukey test analysis are shown in the Supplemental Table S2, for the indicated comparisons between groups of mice. Finally, serum ascorbate levels in 4-mo-old mice correlated significantly with the maximum lifespan of each group of mice in our cohort (Fig. 1C).
Because patients with WS exhibit a lack of growth spurt during adolescence (27), we next followed the total body weight of male mice for the first 16 wk of life. On average the Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate were 15% smaller than the WT, Wrn∆hel/∆hel mice, and 0.01% ascorbate–treated Gulo−/− mice (Fig. 1D). There was no significant difference in body weight between WT, Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice. When Wrn∆hel/∆hel/Gulo−/− mice were treated with 0.4% ascorbate, total body weight was 17% higher than the WT mice at 16 wk of age (Fig. 1E). To determine whether Wrn∆hel/∆hel/Gulo−/− mice were eating less with 0.01% ascorbate, we measured the daily food intake of our mice from each genotype for 1 wk at the age of 4 mo. As indicated in Fig. 1F, the Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate ate less than WT, Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice. The food intake was similar to those 3 groups when Wrn∆hel/∆hel/Gulo−/− mice received 0.4% ascorbate in the drinking water. We also measured the amount of water consumed by these mice for 1 wk at 4 mo of age. Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate drank less water every day than WT and Wrn∆hel/∆hel mice (Fig. 1G). When the water was supplemented with 0.4% ascorbate, Wrn∆hel/∆hel/Gulo−/− mice drank significantly more water every day than WT mice (Fig. 1G). Finally, we determined that there was no significant correlation between food intake and total body weight or between water consumption and total body weight in our cohorts of mice. The Pearson’s correlation coefficient r was 0.7395 (P = 0.15, not significant) for food intake vs. total body weight and 0.7031 (P = 0.19, not significant) for water consumption vs. total body weight. These results indicate that food intake and water consumption were not proportional to the different mean total body weight observed between the genotypes.
The heart, liver, spleen, and visceral fat were also weighed. As indicated in the Supplemental Fig. S1, the liver and heart did not show a significant difference between the different genotypes at 4 mo of age. There was no sign of cardiac or liver fibrosis in 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate like the age-matched WT mice (data not shown). The spleen of 4-mo-old Wrn∆hel/∆hel mice was bigger than the spleen of WT mice. There was a tendency for the spleen to be larger in Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate than in WT mice of the same age (Supplemental Fig. S1). The spleen of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate was significantly bigger than WT mice. The weight of visceral fat was significantly lower in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate than in all the other groups of mice. Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate exhibited a significant increase in visceral fat weight compared with 0.01% ascorbate–treated Wrn∆hel/∆hel/Gulo−/− mice, similar to weights in the other groups of mice (Supplemental Fig. S1).
Overall, our results indicate that Wrn∆hel/∆hel/Gulo−/− mice had a reduced lifespan and were smaller than WT, Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice when they were treated with a minimum of 0.01% ascorbate in drinking water. The supplementation of water with 0.4% ascorbate significantly increased the lifespan and the body weight of the Wrn∆hel/∆hel/Gulo−/− mice.
Impact of 0.01 and 0.4% ascorbate supplementation on WrnΔhel/Δhel/Gulo−/− mouse fertility
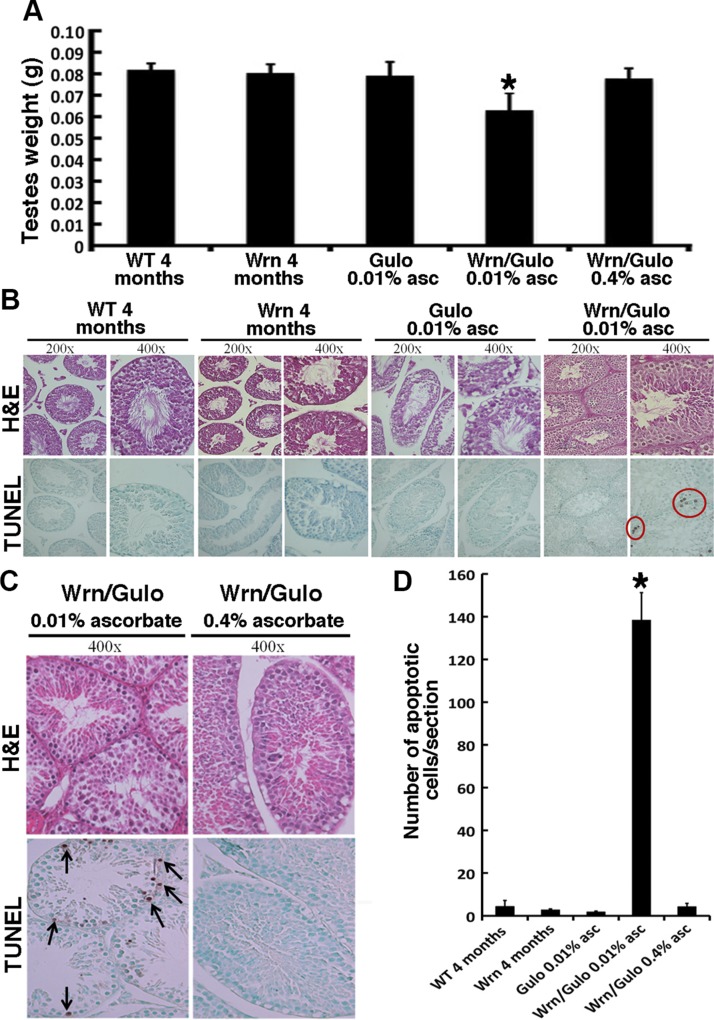
Mating between Wrn∆hel/∆hel/Gulo−/− males and females was unsuccessful in producing offspring when drinking water was supplemented with 0.01% ascorbate. However, when drinking water was supplemented with 0.4% ascorbate at weaning, adult Wrn∆hel/∆hel/Gulo−/− mice produced offspring. Testes from each genotype were weighed at 4 mo of age and Wrn∆hel/∆hel/Gulo−/− males exhibited a 25% decrease in size compared with WT, Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice (Fig. 2A). Note that the testes’ wet weight did not correlate with the total body weight observed for the different genotypes at 4 mo of age (Pearson’s correlation r = 0.6493; P = 0.24). The addition of 0.4% ascorbate in drinking water re-established the normal (WT) size of Wrn∆hel/∆hel/Gulo−/− male testes. We performed histologic examination of the testes of Wrn∆hel/∆hel/Gulo−/− males treated with 0.01% ascorbate and all anatomic structures (spermatids and Leydig and Sertoli cells) looked intact (Fig. 2B). Closer histologic examination of testicular sections at 4 mo of age by TUNEL assay revealed a major increase in overall cell death in Wrn∆hel/∆hel/Gulo−/− mice compared with WT, Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice. Cell death was significantly reduced in Wrn∆hel/∆hel/Gulo−/− males treated with 0.4% ascorbate (Fig. 2C, D).
Effect of ascorbate on testicular morphology of WrnΔhel/Δhel/Gulo−/− mice. A) Testicular wet weight of mice (n = 10) at 4 mo of age. *P < 0.019 vs. all other groups of mice (by post-ANOVA Tukey test). B) H&E staining and TUNEL assay of seminiferous tubules of WT, WrnΔhel/Δhel, Gulo−/−, and WrnΔhel/Δhel/Gulo−/− mice at 4 mo of age. Apoptotic cells are circled in red. C) H&E staining and TUNEL assay of seminiferous tubules of WrnΔhel/Δhel/Gulo−/− mice at 4 mo of age treated with 0.01 or 0.4% ascorbate. Apoptotic cells contain a brown precipitate (arrows). D) Average number of apoptotic cells per histologic section of testes from WT (n = 3), WrnΔhel/Δhel (n = 4), Gulo−/− mice treated with 0.01% ascorbate (n = 4), WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate (n = 6), and WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (n = 10) at 4 mo of age. *P < 0.001 vs. all other groups of mice (by Tukey test). Bars in graphs represent sem. Groups of mice are labeled as in Fig. 1B.
Finally, Wrn∆hel/∆hel/Gulo−/− males treated with 0.01% ascorbate were crossed to WT females but did not produce vaginal plugs underlying other unknown physiologic problems during breeding. Adding 0.4% ascorbate in the drinking water reversed this breeding defect. Wrn∆hel/∆hel/Gulo−/− females treated with 0.01% ascorbate were crossed to WT males. Although we could detect vaginal plugs, these females did not produce offspring. We performed histologic examination of the ovaries from Wrn∆hel/∆hel/Gulo−/− adult females treated with 0.01% ascorbate and did not find any obvious anatomic abnormality (data not shown).
WrnΔhel/Δhel/Gulo−/− mice exhibit bone structure anomalies
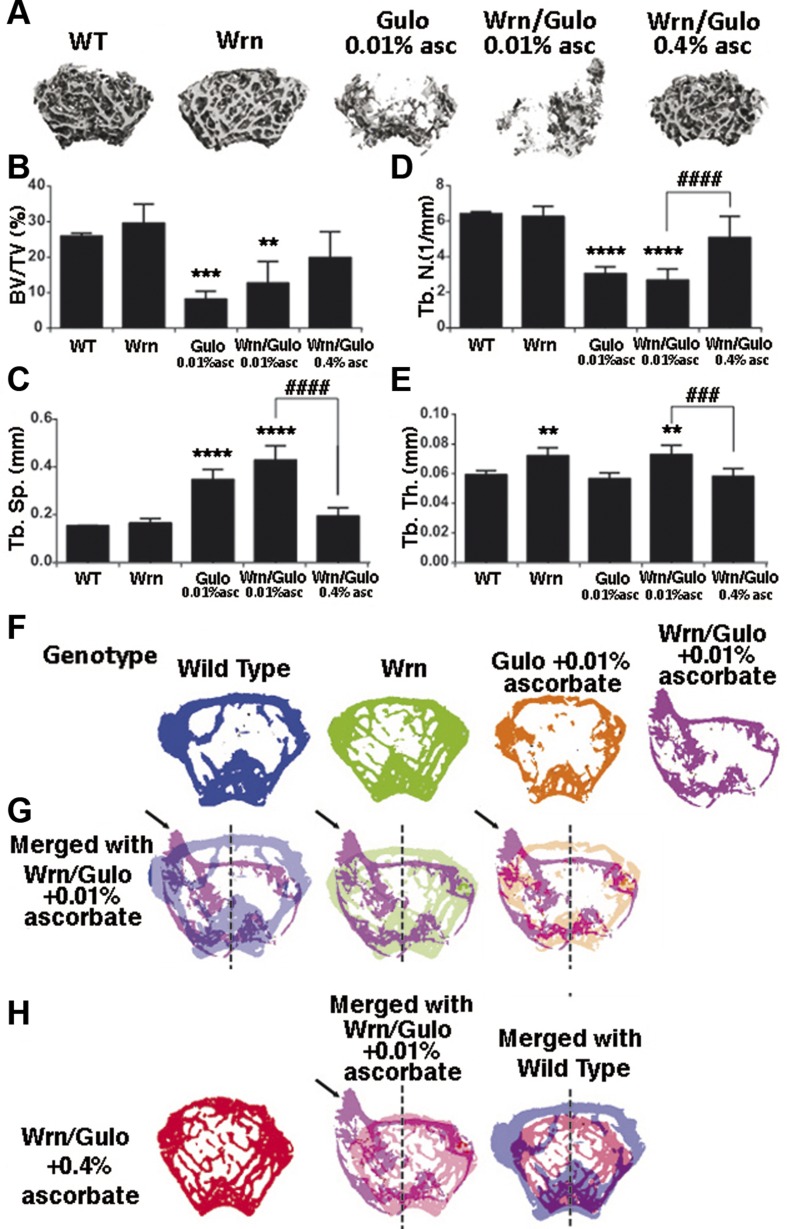
Osteoporosis of the limbs has been described for patients with WS (27). We thus examined the bone structure of hind limbs in our mice. Upon tissue harvesting for histologic analysis, we found the hind limb femurs of Wrn∆hel/∆hel/Gulo−/− mice to break more easily than those of WT mice. We performed micro-CT analysis of the femurs of 3-mo-old males from each genotype. Figure 3A shows representative images of the trabecular ROI for each of the genotypes. The bone volume decreased significantly in Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with WT and Wrn∆hel/∆hel mice. Supplementation of 0.4% ascorbate to Wrn∆hel/∆hel/Gulo−/− mice reversed bone volume to WT levels (Fig. 3B). The decrease in bone volume was accompanied by a significant decrease in trabecular number with a commensurate increase in trabecular separation in Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate (Fig. 3C, D). Trabecular thickness increased significantly in Wrn∆hel/∆hel and 0.01% ascorbate–treated Wrn∆hel/∆hel/Gulo−/− mice (Fig. 3E) compared with WT and 0.01% ascorbate–treated Gulo−/− mice. All these phenotypes in Wrn∆hel/∆hel/Gulo−/− mice were reversed by the supplementation of the drinking water with 0.4% ascorbate.
Impact of ascorbate on the hind limb bone structures of WrnΔhel/Δhel/Gulo−/− mice at 3 mo of age. A) Representative 3-dimensional reconstructions of the trabecular region of interest. B) Trabecular bone volume per total bone volume (%). C) Trabecular separation. D) Trabecular number. E) Trabecular thickness. F) Representative cross-sectional images of distal femurs from the indicated genotypes are shown. G) Cross-sectional images of femurs from WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate since weaning are overlaid onto WT, WrnΔhel/Δhel, and Gulo−/− mutant slices to illustrate bony abnormalities. Note the unusual distribution of trabecular bone (sequestration of bone toward the left quadrants), and misshapen cortical shell (arrow). H) Cross-sectional images of femurs from WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate overlaid onto femurs from WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate since weaning and femurs from untreated WT mice. Note that the aberrant features of the WrnΔhel/Δhel/Gulo−/− mutant are absent with the 0.4% ascorbate treatment. Labels in each panel: WT, wild type; Wrn, WrnΔhel/Δhel mice; Gulo, Gulo−/− mice; Wrn/Gulo, WrnΔhel/Δhel/Gulo−/− mice. Bars in graphs represent sem. **P > 0.01; ***P > 0.005; ****P > 0.0001 vs. WT; ###P > 0.005; ####P > 0.0001 Wrn/Gulo 0.01% asc vs. Wrn/Gulo 0.01% asc (by 1-way ANOVA, with Sidak’s multiple comparisons post hoc test).
Cross-sectional images of Wrn∆hel/∆hel/Gulo−/− femurs were overlaid onto WT, Wrn∆hel/∆hel, and Gulo−/− mutant slices to illustrate bony abnormalities (Fig. 3F–H). There was an unusual distribution of trabecular bone (sequestration of bone toward the left quadrants) and misshapen cortical shell in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate (Fig. 3G). These aberrant features in the Wrn∆hel/∆hel/Gulo−/− mice were absent with 0.4% ascorbate (Fig. 3H).
Metabolomic profiling of mutant mice
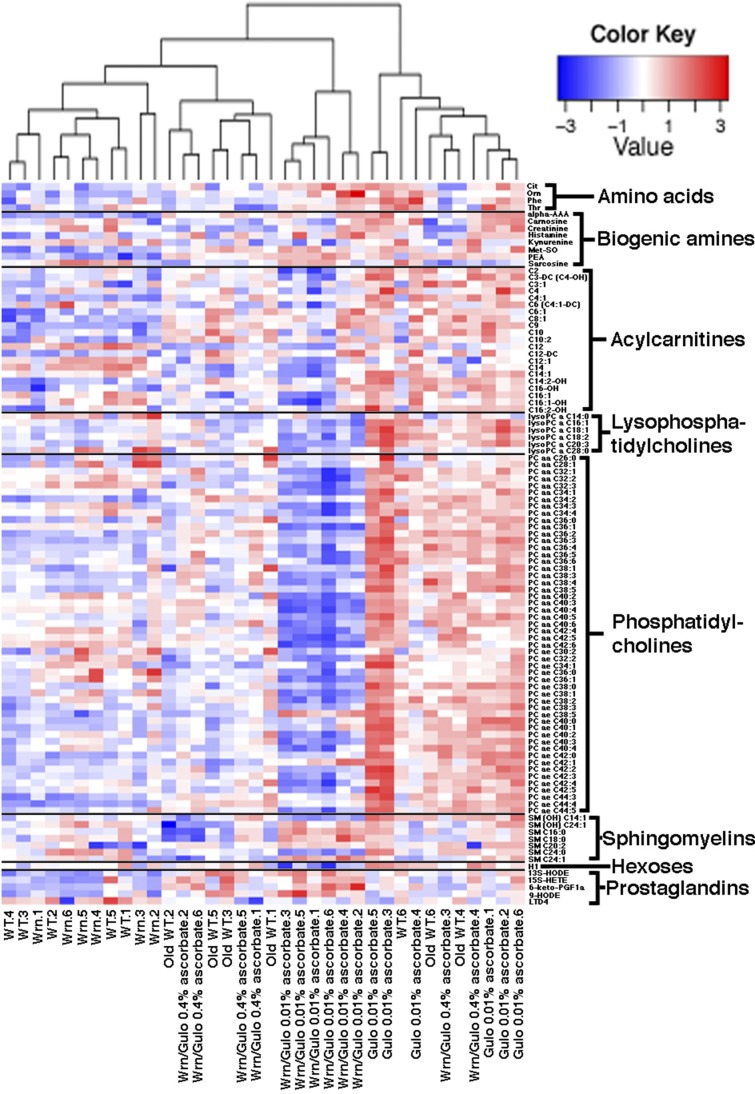
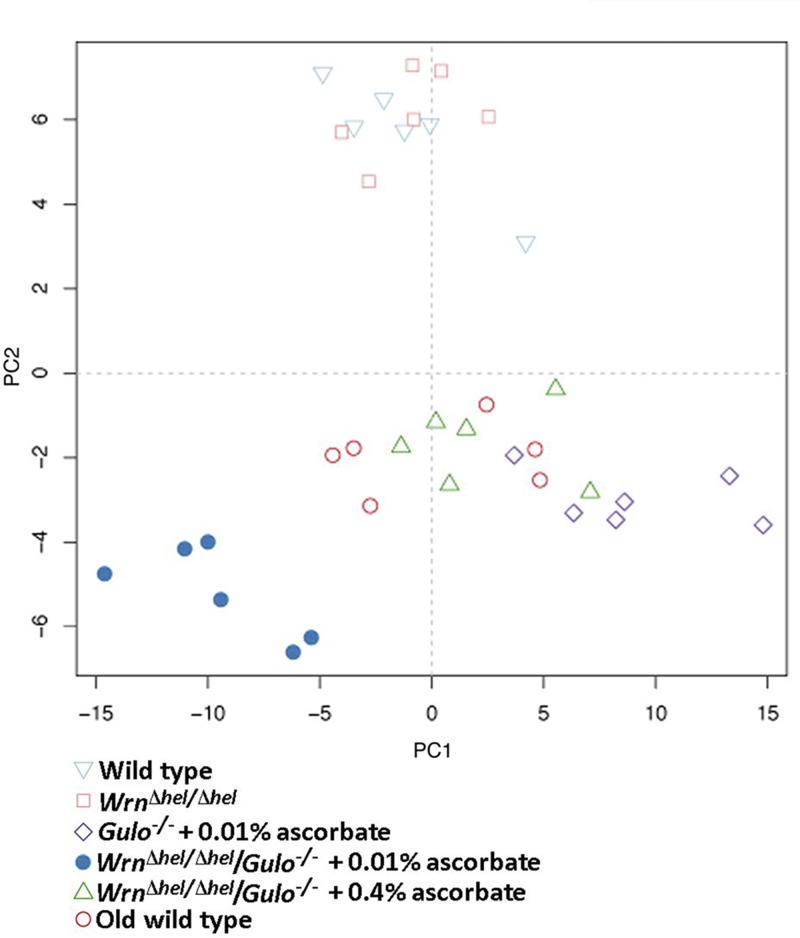
As ascorbate has an impact not only on the lifespan of Wrn∆hel/∆hel/Gulo−/− mice but also on their total visceral fat depots, we next examined 203 metabolites (including different lipid species) in the serum of the mouse cohorts described in Fig. 1B using a targeted MS approach. The serum metabolite concentrations in 6 males of each group are shown for all the metabolites in Supplemental Data S1. To identify the metabolites significantly altered between mouse cohorts, a nonparametric Kruskal-Wallis test was applied to the Supplemental Data S1. A summary of the metabolites significantly altered (P < 0.01) in at least 1 of the cohorts is given in a form of a heat map in Fig. 4. Changes were indicated as a z-score value for each metabolite in the serum of each animal. The heat map revealed 104 metabolites that significantly differed between groups (Fig. 4). These included 52 phosphatidylcholines, 6 lysophosphatidylcholines, 21 acylcarnitines, 8 biogenic amines, 5 prostaglandins, 7 sphingomyelins, hexoses, and 4 aa. The dendrogram on top of the heat map indicated that the 4-mo-old Wrn∆hel/∆hel and the WT mice were clustered together. Wrn∆hel/∆hel/Gulo−/− mice given 0.01% ascorbate in their drinking water formed a distinct group. Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate in their drinking water formed other clusters with the old WT mice. We further explored the metabolic profile of each group by employing an unsupervised PCA on all the 203 metabolites (PCA in Fig. 5). The WT and the Wrn∆hel/∆hel mice clustered together in the middle of the upper part of the graph. The Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate clustered in the lower left quadrant of the graph. The Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate formed a cluster with the old WT (20-mo-old) mice in the middle of the lower part of the graph. Gulo−/− mice treated with 0.01% ascorbate clustered in the lower right quadrant of the graph. These results indicate that the overall metabolic profile of 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate was very different from the other genotypes. The metabolic profile of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate resembled the metabolic profile of 20-mo-old WT mice.
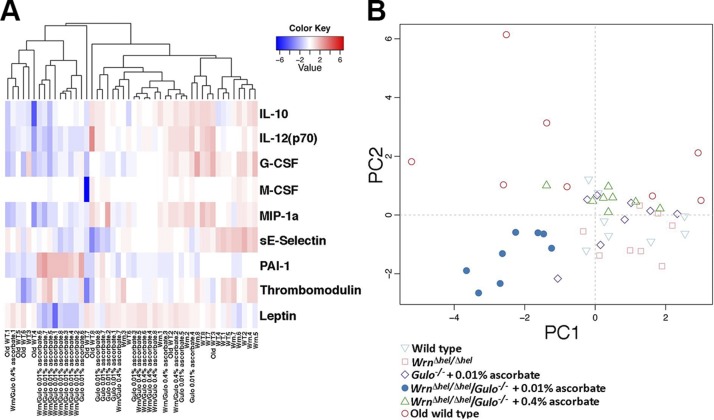
Heat map depicting the z score of the log base 10 in serum metabolites concentration (rows) between individual (columns) WT and mutant mice treated with different amounts of ascorbate. Columns are reordered by hierarchical clustering using the genotype and ascorbate treatments. Metabolites are grouped according to chemical classification. Young (4 mo) WT mice are labeled WT.i (where i = 1–6). Old (20 mo) WT mice are labeled Old WT.i. WrnΔhel/Δhel mice are labeled Wrn.i. Gulo−/− mice treated with 0.01% ascorbate are labeled Gulo 0.01% ascorbate.i. WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate are labeled Wrn/Gulo 0.01% ascorbate.i. WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate are labeled Wrn/Gulo 0.4% ascorbate.i. The Euclidian distance and complete agglomerative methods were used for clustering.
Inflammatory and cardiometabolic profiles of mutant mice
As metabolite disturbances can lead to an inflammatory response or changes in cardiovascular risk factors (22), we measured the levels of 42 cytokines/chemokines, metabolic hormones, and cardiovascular risk factors in the serum of our different mouse cohorts. Serum cytokine concentrations in 8 males of each group are shown in the Supplemental Table S1. To identify the cytokines significantly altered in either mouse groups, a nonparametric Kruskal-Wallis test was applied to the data. A summary of the cytokines significantly altered in at least one of the groups is given in a form of a heat map in Fig. 6A. Changes were indicated as a z-score value for each cytokine in the serum of each animal. The dendrogram on top of the heat map indicated 2 major leaves. The young WT and the Wrn∆hel/∆hel mice tended to cluster together. All the Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate clustered together. The old WT, the Gulo−/− mice treated with 0.01% ascorbate, and the Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate were distributed unequally within the 2 major leaves of the dendrogram. To get a better picture for each group of mice, we performed an unsupervised PCA using data from all the cytokines (Fig. 6B). The PCA approach showed that all Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate were clustered in the lower left quadrant of the graph. The young (4 mo) WT mice were mainly clustered in the right quadrants of the graph with the Wrn∆hel/∆hel mice. The old (20 mo) WT mice were distributed in the upper quadrants and showed a large variability between individuals. The Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate were also clustered in the upper quadrants of the graph and showed less variability between individuals. The Gulo−/− mice treated with 0.01% ascorbate were distributed in all 4 quadrants of the graph. To summarize the major finding of this PCA data, the serum cytokine profile of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate was unique in our cohorts of mice and did not overlap with any other mouse groups.
Impact of ascorbate and genotype on the cytokinome of mice. A) Heat map depicting the z score of log base 10 in serum cytokine concentrations (rows) between individual (columns) WT and mutant mice treated with different amounts of ascorbate. Significantly altered cytokines with a value of P < 0.01 are shown. Columns are reordered by hierarchical clustering by using the genotype and ascorbate treatments. The Euclidian distance and complete agglomerative methods were used for clustering. B) PCA graph demonstrating the differentiation effect of ascorbate and genotype on the cytokinome profiles of mice. The labels and symbols on the heat map and the PCA graph are identical to Figs. 4A and and5,5, respectively.
The heat map revealed 9 cytokines or factors that significantly differed between groups (Fig. 6A). Graphs representing the levels of secreted factors in each group are shown in Supplemental Fig. S2. The most marked findings were a decreased in IL-12 (p70), G-CSF, MIP-1α, soluble E-selectin, and leptin in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate than in WT mice. PAI-1 increased in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with WT mice. Treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate reversed the IL-12 (p70), G-CSF, MIP-1α, leptin, and PAI-1 phenotypes (Supplemental Fig. S2).
Ratios of specific metabolites indicate various pathway anomalies in WrnΔhel/Δhel/Gulo−/− mice
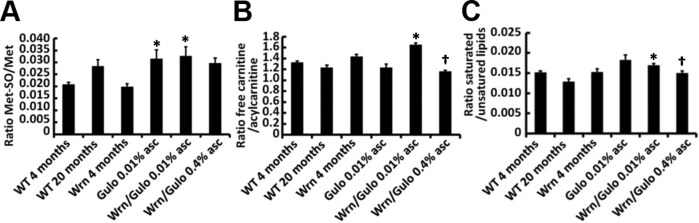
Figure 7 provides metabolite ratios that showed a significant difference between groups of mice. Methionine-sulfoxide (Met-SO) can be generated via a 2-electron–dependent mechanism and the Met-SO:Met ratio in the serum can be regarded as a marker of oxidative stress (28). As indicated in Fig. 7A, the Met-SO:Met ratio was significantly higher in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate than in WT mice, indicating an increase oxidative stress in these mice. Wrn∆hel/∆hel/Gulo−/− mice given supplemental 0.4% ascorbate only slightly, but not significantly, decreased this ratio.
Ratios of metabolite significantly altered in WrnΔhel/Δhel/Gulo−/− mice. A) Met-SO:Met ratio. *P = 0.046 vs. WT mice (by post-ANOVA Tukey test). B) Free carnitine (C0):acylcarnitine ratio. *P < 0.012 vs. all other groups of mice and †P = 0.001 vs. WrnΔhel/Δhel/Gulo−/− mice given 0.01% supplemental ascorbate (by Tukey test). C) Saturated:unsaturated phosphatidylcholines ratio. Groups of mice are labeled as in Fig. 1B. *P = 0.021 vs. WT mice and †P = 0.027 vs. WrnΔhel/Δhel/Gulo−/− mice given 0.01% supplemental ascorbate (by unpaired Student t tests). Bars represent sem.
Acylcarnitines are important molecules for mitochondrial function and lipid metabolism. As indicated in Fig. 4, 21 acylcarnitine species were significantly different among our different cohorts of mice. We examined the ratio of free carnitine over the sum of these 21 acylcarnitine species. As indicated in Fig. 7B, this ratio increased significantly in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with all the other groups of mice. Wrn∆hel/∆hel/Gulo−/− mice given supplemental 0.4% ascorbate significantly reversed this ratio to WT. We also examined the ratio of saturated to unsaturated phosphatidylcholine species. We pooled the serum concentrations of saturated phosphatidylcholines and of unsaturated phosphatidylcholines to calculate a ratio. [For example, the concentrations of PC diacyl (PC aa) C26:0, PC aa C36:0, PC ae C36:0, PC ae C38:0, PC ae C40:0, and PC ae C42:0 that are significantly changed in our mouse cohorts based on Fig. 4 were summed to obtain the numerator of the ratio]. As indicated in Fig. 7C, the ratio of saturated:unsaturated phosphatidylcholines was not significantly different between groups, according to results of ANOVA. However, if we compared only the 0.01% ascorbate–treated Wrn∆hel/∆hel/Gulo−/− mice with the WT mice, an unpaired Student t test showed a significant difference. Wrn∆hel/∆hel/Gulo−/− mice given supplemental 0.4% ascorbate significantly reversed this ratio to WT levels.
Oxidative stress in the liver tissue of WrnΔhel/Δhel/Gulo−/− mice
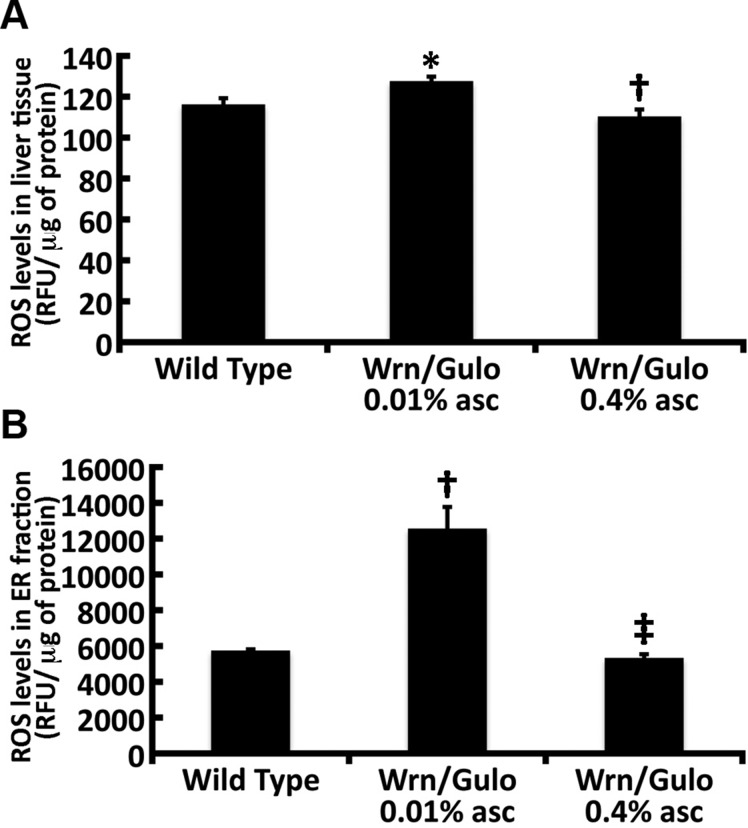
We had found that the liver of Wrn∆hel/∆hel and Gulo−/− mice treated with low amounts of ascorbate (<0.01% in drinking water) exhibit an oxidative stress in the ER (11, 21, 29). The liver plays a pivotal role in nutrient, hormone, lipid, and metabolic waste product processing, thereby maintaining body homeostasis (30). The liver ER is the normal location of ascorbate synthesis in mice (31). Furthermore, the active catalytic site of the membrane-associated Gulo enzyme is located in the ER lumen (32). We did not detect hepatic steatosis or significant difference in fat content between Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with age-matched (4-mo-old) mice of the other groups (data not shown). However, we observed a significant increase in the mitochondrial DNA mutation rate in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with age-matched (4-mo-old) WT mice (Table 1). It was comparable to older (20-mo-old) WT mice. Treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate in drinking water decreased the mutation rate to young WT level (Table 1). The mutation rate in mitochondrial DNA of Wrn∆hel/∆hel and Gulo−/− mice treated with 0.01% ascorbate was not significantly different from that of age-matched WT mice. We next focused on reactive oxygen species (ROS) levels in the liver of Wrn∆hel/∆hel/Gulo−/− mice and age-matched WT mice. We quantified ROS levels in total liver tissues of WT and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 or 0.4% ascorbate. ROS levels in total liver lysate from Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate increased significantly (∼10%) compared with age-matched WT mice (Fig. 8A). Treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate significantly decreased total ROS in the liver by ∼16%.
TABLE 1
Effect of the genotype and ascorbate on mitochondrial mutation rate
| Genotype | Mutation/sequence (bp)a | Percentage | Pb | ||||
|---|---|---|---|---|---|---|---|
| WrnΔhel/Δhel | Gulo−/− 0.01% ascorbate | WrnΔhel/Δhel/Gulo-/- 0.01% ascorbate | WrnΔhel/Δhel/Gulo-/- 0.4% ascorbate | Old WT (20 mo) | |||
| WT (4 mo) | 19/19,693 | 0.096 | 0.3101 | 0.2516 | 0.1238 | 0.1061 | 0.0340* |
| WrnΔhel/Δhel | 17/21,039 | 0.081 | — | 0.5000 | 0.0381* | 0.2312 | 0.0074* |
| Gulo−/− 0.01% ascorbate | 16/21,035 | 0.076 | |||||
| Gulo−/− | — | — | 0.0259* | 0.2875 | 0.0046* | ||
| WrnΔhel/Δhel/Gulo−/− 0.01% ascorbate | 29/20,514 | 0.141 | — | — | — | 0.0059* | 0.3042 |
| WrnΔhel/Δhel/Gulo−/− 0.4% ascorbate | 12/19,981 | 0.060 | — | — | — | — | 0.0011* |
| Old WT (20 mo) | 32/19,229 | 0.170 | — | — | — | — | — |
ROS levels in total liver extract and the ER enriched fraction of 4-mo-old WrnΔhel/Δhel/Gulo−/− mice. A) ROS levels in total liver extract. *P < 0.05 vs. WT mice and †P = 0.01 vs. WrnΔhel/Δhel/Gulo−/− mice given 0.01% supplemental ascorbate, by Tukey test. B) ROS levels in the ER enriched fraction of WT and WrnΔhel/Δhel/Gulo−/− mice given drinking water supplemented with 0.01 or 0.4% ascorbate. †P < 0.01 vs. WT mice; ‡P = 0.01 vs. WrnΔhel/Δhel/Gulo−/− mice given 0.01% supplemental ascorbate (by Tukey test). ROS was detected with DCFA and is shown as relative fluorescence units (RFU). Bars in each graph represent sem.
We previously found that Wrn∆hel/∆hel mice exhibited a significant increase (∼15%) in ROS levels in the ER fraction of their liver compared with WT mice (29). We next examined ROS levels in the ER fraction of liver from our Wrn∆hel/∆hel/Gulo−/− mice. ROS level in the ER fraction of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate increased significantly (2.2-fold) compared with that in age-matched WT mice (Fig. 8B). Treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate significantly decreased ER ROS to WT levels. These results indicate that 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate exhibit greater oxidative ER stress levels than Wrn∆hel/∆hel mice (29). We thus examined different ER stress markers in our WT and Wrn∆hel/∆hel/Gulo−/− mice. PERK and IRE1α were first analyzed by Western blot analysis in ER-enriched fractions (33). Calreticulin, a protein associated with the ER, was used as a loading control. The PERK protein level increased significantly in the liver ER of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with WT mice (Fig. 9A, B). Treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate significantly decreased PERK to the WT level (Fig. 9B). IRE1α protein increased significantly in the liver ER enriched fraction of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 and 0.4% ascorbate compared with age-matched WT mice (Fig. 9A, C). There was no significant difference between the effects of the 0.01 and 0.4% ascorbate treatments in WrnΔhel/Δhel/Gulo−/− mice.
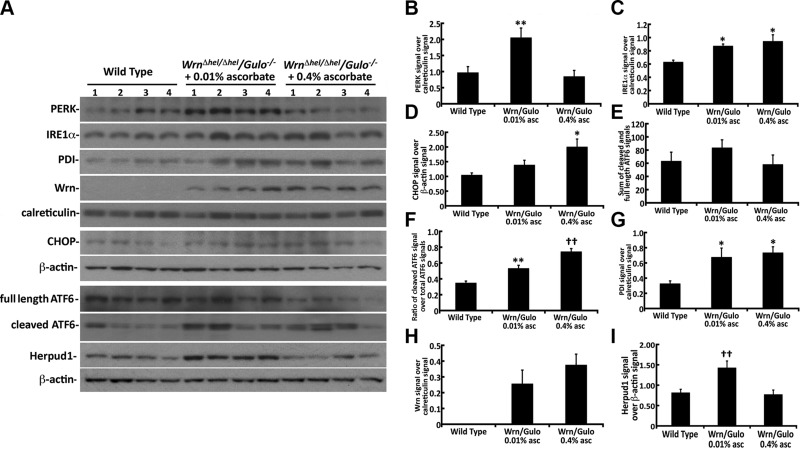
Activation of ER stress protein markers in the liver of 4-mo-old WrnΔhel/Δhel/Gulo−/− mice. A) Western blots for the indicated proteins in 4-mo-old WT and WrnΔhel/Δhel/Gulo−/− mice given supplemental 0.01 or 0.4% ascorbate. B) Ratio of total PERK signal over calreticulin signal in ER enriched fractions from the Western blots. **P < 0.05 compared with WT and WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (by Tukey post-ANOVA test). C) Ratio of total IRE1α signal over calreticulin signal in ER enriched fractions from the Western blots. *P < 0.05 compared with WT mice (by Tukey post-ANOVA test). D) Ratio of CHOP signal over β-actin signal in total liver lysates from the Western blots *P < 0.05 compared with WT mice (by Tukey post-ANOVA test). E) Sum of the cleaved and full-length ATF6 signals in total liver extracts. F) Ratio of the cleaved over total (cleaved and full length) ATF6 signals in total liver extracts. **P < 0.01 compared with WT mice; ††P < 0.01 compared with WT and WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate (by Tukey post-ANOVA test). G) Signal of PDI signal over calreticulin signal in ER enriched fractions from the Western blots. *P < 0.05 compared with WT mice (by Tukey post-ANOVA test). H) Ratio of mutant WrnΔhel signal over calreticulin signal in ER enriched fractions from the Western blots. I) Ratio of HERPUD1 signal over β-actin signal in total liver lysates from the Western blots. ††P < 0.01 compared with WT and WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (by Tukey post-ANOVA test). Bars in all histograms represent SEM of 4 mice.
The PERK and IRE1α pathways are known to activate the transcription of CHOP (34). Since CHOP is not found in the ER, proteins from total liver lysates were analyzed by Western blot. β-actin was used as a loading control. There was only a tendency for CHOP to be increased in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with age-matched WT mice. By contrast, CHOP protein level increased significantly (∼2-fold) in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate compared with age-matched WT mice (Fig. 9A, D).
Upon ER stress, the ER membrane-associated ATF6 protein is cleaved into a subunit that translocates into the nucleus to activate the transcription of target genes (33). We thus examined the levels of full length and cleaved ATF6 in total liver extracts (Fig. 9A, E, F). There was only a tendency for the sum of full-length and cleaved ATF6 signals to be higher in the liver of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate than in the liver of WT and WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (Fig. 9E). However, when we calculated the ratio of cleaved ATF6 over the sum of full-length and cleaved signals, the levels of cleaved ATF6 was higher in the WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate than in WT mice. The levels of cleaved ATF6 were even higher in the liver of WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (Fig. 9F).
We have reported that the mutant Wrn protein (WrnΔhel) is mislocalized in the cytoplasm of Wrn∆hel/∆hel mice (11). Further microscopy analyses indicated a colocalization of WrnΔhel proteins with the PDI. We examined the levels of PDI in our mouse cohort. As indicated in Fig. 9A, G, the levels of PDI increased significantly in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 and 0.4% compared with untreated WT mice. This PDI increase in the liver ER fraction of Wrn∆hel/∆hel/Gulo−/− mice corresponded to the presence of WrnΔhel protein in this ER fraction (Fig. 9A, H). No normal Wrn protein was detected in the ER fraction of WT mice. As previously found with Wrn∆hel/∆hel mice (29), the treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate in drinking water did not decrease the expression level of the mutant WrnΔhel protein (Fig. 9H).
Total eIF2α, ATF4, glucose-regulated protein–78, and HSC70 protein levels were not different between WT and WrnΔhel/Δhel/Gulo−/− mice treated with different concentrations of ascorbate in drinking water (Supplemental Fig. S3). Phosphorylation of eIF2α was not significantly different between WT and ascorbate treated WrnΔhel/Δhel/Gulo−/− mice (Supplemental Fig. S3).
We also analyzed ATF4, CHOP, HSC70, and full-length and cleaved ATF6 protein levels in age-matched WT, Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice, because it had not been examined in our other studies (11, 21, 29). There was no significant difference in CHOP, HSC70, or full-length and cleaved ATF6 protein levels between these groups of mice, according to ANOVA results (Supplemental Fig. S4). ATF4 was lower in Wrn∆hel/∆hel, and 0.01% ascorbate–treated Gulo−/− mice compared with WT mice, but it was not significant according to ANOVA.
The ER-associated degradation pathway is increased in the liver tissue of WrnΔhel/Δhel/Gulo−/− mice
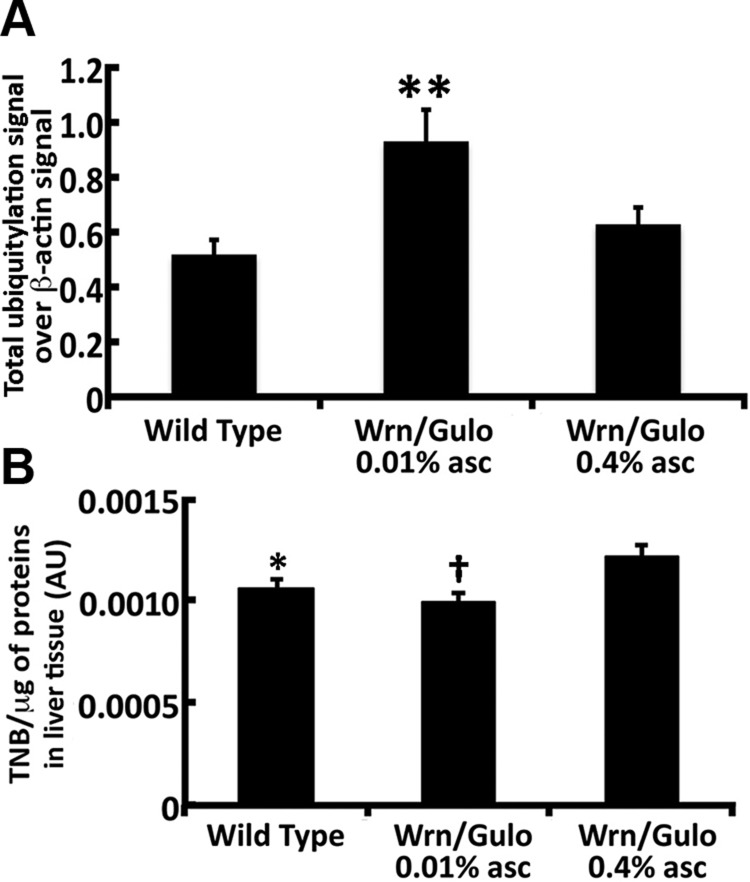
To eliminate misfolded proteins from the ER, cells rely on ubiquitin-proteasome–dependent ER-associated degradation (ERAD). Proteolysis of ERAD substrates by the proteasome requires their ubiquitylation and retrotranslocation from the ER to the cytoplasm (35). Homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member (HERPUD)-1 protein facilitates ERAD. We thus examined the levels of HERPUD1 in the liver of WT and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 and 0.4% ascorbate. The HERPUD1 protein levels were significantly increased in the total lysates of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate (Fig. 9A, I). Treating Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate significantly decreased HERPUD1 to WT levels. We next examined the levels of total protein ubiquitylation in the liver of WT and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 and 0.4% ascorbate by Western blots. Total β-actin was used as a loading control (Supplemental Fig. S5). As indicated in Fig. 10A, total protein ubiquitylation levels increased significantly in the total lysates of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate. Treating Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate significantly decreased total ubiquitylation to WT levels. In contrast, Gulo−/− mice treated with 0.01% ascorbate showed a significant decrease in HERPUD1 levels compared with age-matched WT mice (Supplemental Fig. S4A, C). The level of total protein ubiquitylation in the liver of Gulo−/− mice treated with 0.01% ascorbate tended to decrease compared with that in WT mice but it was not significant according to ANOVA results (Supplemental Fig. S4A, B). HERPUD1 protein and total ubiquitylation levels were not different between untreated Wrn∆hel/∆hel and age-matched WT mice (Supplemental Fig. S4A–C). Overall, these results indicate that untreated Wrn∆hel/∆hel mice and Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate showed very different HERPUD1 and ubiquitylation levels in their liver.
Sulfhydrylation and ubiquitylation modifications of proteins in the liver of 4-mo-old WrnΔhel/Δhel/Gulo−/− mice. A) Ratio of total ubiquitylated proteins signal over β-actin signal from the Western blots. **P < 0.05 compared with WT and WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (by Tukey post-ANOVA test). Bars in all histograms represent sem of 4 mice. B) Quantification of thiol groups on proteins extracted from the liver of WT and WrnΔhel/Δhel/Gulo−/− mice treated with 0.01 and 0.4% ascorbate by the DTNB assay. *P < 0.05; †P < 0.01 compared with WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate (by Tukey post-ANOVA test).
Because we detected increased ROS in the liver tissues of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate, we hypothesized that such ROS would react directly with the cysteine thiols of unfolded proteins or nascent peptides during protein synthesis to convert them to sulfenic acids (36). We thus extracted proteins from the liver tissues of WT and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 or 0.4% ascorbate and measured the levels of intact thiols on proteins with the DTNB reagent (26). The levels of intact thiols on proteins were significantly lower in WT and much lower in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% than in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate (Fig. 10B) . Overall, these results indicate that the presence of the WrnΔhel mutant protein in the liver of Wrn∆hel/∆hel/Gulo−/− mice affected the levels of intact thiols on proteins in general, as well as the amount of ubiquitylated proteins. Treatment of Wrn∆hel/∆hel/Gulo−/− mice with 0.4% ascorbate improved these phenotypes.
DISCUSSION
Mouse model
We possess in our laboratory a mouse line bearing a deletion of part of the helicase domain in the WRN gene ortholog (10). Such WrnΔhel/Δhel mice develop metabolic abnormalities and age-related pathologies leading to a shorter mean lifespan compared with WT mice (10, 11, 29, 37). The phenotypes associated with these mice are improved with a 0.4% ascorbate treatment in drinking water (15, 19, 29). These results indicated that, even though, unlike humans (31), mice can synthesize their own ascorbate, additional ascorbate in the diet was necessary to improve the span of good health in this mouse model. To better evaluate the impact of different doses of ascorbate on the longevity and the pathophysiological phenotypes of our Wrn mutant mice, we crossed the WrnΔhel/Δhel mice with a knockout mouse line that lacks the enzyme required for de novo ascorbate synthesis (encoded by the Gulo gene) (20).
As reported, Gulo−/− mice become rapidly sick without ascorbate in the drinking water but live up to ∼16 mo when treated with 0.01% ascorbate and ∼32 mo when treated with 0.4% ascorbate (21). In contrast, in our study, the maximum lifespan of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate in drinking water was not longer than 8 mo (Fig. 1A). Thus, WrnΔhel/Δhel mice unable to synthesize their own ascorbate showed a major decrease in their lifespan. The maximum lifespan of WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate in drinking water increased significantly, similar to that of the WT cohort (P = 0.137, log rank test). Although the maximum lifespan of WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate increased compared with that of WrnΔhel/Δhel mice, the difference was not significant (P = 0.134, log rank test). This finding suggests that the beneficial effect of 0.4% ascorbate on lifespan is attributable to the pharmacological correction of the Gulo−/− mutation in our mouse model. However, it is possible that our WrnΔhel/Δhel/Gulo−/− cohort treated with 0.4% ascorbate was rather small (n = 14) and therefore did not provide enough statistical power to detect a significant difference when we compared WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate to untreated WrnΔhel/Δhel mice. Nevertheless, the 0.4% ascorbate treatment significantly improved several other abnormal phenotypes (such as body weight, fertility, or osteopenia) exacerbated by the WrnΔhel/Δhel mutation on the Gulo−/− genetic background. Serum ascorbate levels in 4-mo-old mice correlated significantly with the maximum lifespan of each group of mice in our cohort. Also, our results indicated that supplementation of drinking water with 0.01% ascorbate was suboptimal for the span of good health of WrnΔhel/Δhel/Gulo−/− mice compared with Gulo−/− mice.
Unlike Gulo−/− mice, WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate were sterile, an effect encountered in some patients with WS (27). We could not detect specific anomalies with the ovaries of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate. In contrast, the testicular weight of WrnΔhel/Δhel/Gulo−/− males treated with 0.01% ascorbate was significantly lower compared with that of Gulo−/−, WrnΔhel/Δhel, and WT mice and this difference was independent of total body weight in our different mouse cohorts. The number of apoptotic figures revealed by the TUNEL assay indicated a significant increase in spermatogonia mortality in the testes of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate. No information is available with regard to the prevalence of apoptotic cells in testes of patients with WS but it has been reported that aged men (70–89-yr-old) present a significant augmentation in apoptotic signals located in the nuclei of spermatogonia and primary spermatocytes compared with young men (24–40-yr-old) (38). The 0.4% ascorbate treatment reversed the testicular phenotype of WrnΔhel/Δhel/Gulo−/− mice and they were successful in producing offspring.
Because 66% of clinically confirmed WS patients develop osteoporosis at a young age (∼35-yr of age) (39, 40), the bone structure in the limbs of mice was examined with a microcomputed tomography scan (24). The bone volume decreased significantly in Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with WT and Wrn∆hel/∆hel mice. The decrease in bone volume was accompanied by a significant decrease in trabecular number with a commensurate increase in trabecular separation in Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate. Cross-sectional images of Wrn∆hel/∆hel/Gulo−/− femurs overlaid onto WT, Wrn∆hel/∆hel and Gulo−/− mutant slices illustrated gross bony abnormalities, including an unusual distribution of trabecular bone and misshapen cortical shell in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with the other mouse cohorts including the Gulo−/− mice treated with 0.01% ascorbate (Fig. 3). Thus, the abnormal bone structure in Wrn∆hel/∆hel/Gulo−/− mice was more severe than in the other mouse genotypes. Such aberrant osteopenic features of Wrn∆hel/∆hel/Gulo−/− mice were absent upon treatment with 0.4% ascorbate in drinking water.
Body weight and metabolic profile of WrnΔhel/Δhel/Gulo−/− mice
A common feature among patients with WS is their short stature (27). Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate were smaller than all the other groups of mice, including untreated WrnΔhel/Δhel and 0.01% ascorbate–treated Gulo−/− mice (Fig. 1D). However, contrary to what has been described for patients with WS (41), visceral fat weight was significantly lower in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate (Supplemental Fig. S1). Concomitantly, our targeted MS analyses of serum metabolites revealed a decrease in the levels of several lysophosphatidylcholine and phosphatidylcholine lipid types and hexose molecules in WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate compared with levels in the other groups of mice. The serum leptin level in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate was also severely lower than in the other groups of mice (Supplemental Fig. S2). Leptin is an important hormone that regulates food intake according to body fat mass, and its secretion correlates with the size of adipose mass in mice (42–44). Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate ate less than the other groups of mice. Upon 0.4% ascorbate treatment, serum leptin level significantly increased and reached serum concentrations above 7000 pg/ml in Wrn∆hel/∆hel/Gulo−/− mice. A study on serum leptin levels in Gulo−/− mice indicated no significant difference between the 0.01 and 0.4% treatments with concentrations reaching 4000–6000 pg/ml (21). Leptin serum levels are ∼3000–4000 pg/ml in age-matched WT mice (this study and reference 21). In addition, Gulo−/− mice treated with 0.4% ascorbate do not drink or eat more than Gulo−/− mice treated with 0.01% ascorbate (21). These results indicate that Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01 or 0.4% ascorbate behave very differently from Gulo−/− mice.
In addition to lower levels of several lipid types in the serum, Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate exhibited a significant increase in the ratio of serum saturated and unsaturated lipids and the ratio of free carnitine:acylcarnitine compared with WT mice (Fig. 7B, C). Of note, dyslipidemia has been associated with 85% of patients with genetically confirmed WS (40). A decrease in unsaturated lipid species may lead to perturbation of cellular membrane fluidity with age (45). An abnormal increase in saturated lipids, in return, may contribute to the development of inflammatory and oxidative stress (46). Carnitine is necessary for the transport of fatty acids across the mitochondrial membrane for their catabolism during cycles of β-oxidation (47). It is possible that the lower levels of several acylcarnitine molecules (Fig. 4) are imputable to the overall decrease of several lipid types in the serum of Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate. This phenotype was not observed in all the other groups of mice.
As published for the Gulo−/− and WrnΔhel/Δhel mice (21, 29), addition of 0.4% ascorbate to the drinking water also significantly improved the altered metabolic phenotypes observed in WrnΔhel/Δhel/Gulo−/− mice. The PCA clearly indicated that the metabolic profile of 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate clustered together and did not overlap any other mouse group (Fig. 5). The metabolic profile of 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate did not overlap the profile of 4-mo-old WT mice, but it did overlap the profile of 20-mo-old WT mice (Fig. 5). The reason for this overlap in metabolic profiles is unknown. We observed that Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate gained weight more rapidly than age-matched WT mice during the first 10–16 wk of life (Fig. 1E), which certainly affected their overall metabolic profile. In addition, the ratio of visceral fat weight to total body weight was similar between Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate and untreated 20-mo-old WT mice (Supplemental Fig. S1). Finally, the PCA indicated very little overlap between Gulo−/− and Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate. A recently published PCA showed that the metabolic profile of Gulo−/− mice treated with 0.4% ascorbate was similar to that of 4-mo-old Gulo−/− mice treated with 0.01% ascorbate and did not overlap the profile of age-matched untreated WT mice (21) and thus would not overlap that of 20-mo-old WT mice. Future longitudinal studies on the metabolic profiles of these mice will provide a more complete picture of what is happening to these different mouse cohorts with age.
Cytokinome profile of WrnΔhel/Δhel/Gulo−/− mice
Metabolite disturbances can lead to an inflammatory response or changes in cardiovascular risk factors (22), and we therefore measured the levels of 42 cytokines/chemokines, hormones, and cardiovascular risk factors in the serum of our different mouse cohorts. The PCA clearly indicated that the profile of 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate was clustered and did not significantly overlap that of the other mouse groups (Fig. 6B). In contrast to the metabolic profile, the cytokinome profile of 4-mo-old Wrn∆hel/∆hel/Gulo−/− mice treated with 0.4% ascorbate was much closer to the profile of 4-mo-old WT mice than that of 20-mo-old WT mice.
When we examined each analyte, the most marked findings were a significant decrease in IL-12 (p70), G-CSF, MIP-1α, and E-selectin, as well as a significant increase in PAI-1 in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate compared with WT mice. IL-12(p70) promotes the differentiation of type-1 helper T (Th1) cells, thereby supporting cellular immunity (48). It is naturally produced by dendritic cells, macrophages, and neutrophils. G-CSFs and M-CSFs are hematopoietic cytokines that stimulate the bone marrow to produce granulocytes and macrophages for release into the bloodstream (49–51). G-CSF is produced by the endothelium, macrophages, and several other immune cells. M-CSF is produced in the bone marrow and by endothelial cells, fibroblasts, osteoblasts, and smooth muscle cells (52). MIP-1α is produced by macrophages and acts as a chemoattractant to a variety of cells, including monocytes, T cells, B cells, and eosinophils (53). E-selectin plays an important role in recruiting leukocytes to sites of vascular injury and allows leukocytes to tightly bind to the endothelial surface (54). The observed decrease of these cytokines in Wrn∆hel/∆hel/Gulo−/− mice treated with 0.01% ascorbate may underscore a dysfunction of part of the immune system which may be less efficient to respond to tissue damage. Notably, intracellular ascorbate concentration is important for the activity of macrophages (55). A thorough analysis of the immune cells from these mice is necessary to determine which biologic processes are affected by the accumulation of an abnormal Wrn mutant protein in such cell types.
PAI-1 is a serine protease inhibitor that functions as the principal inhibitor of tissue plasminogen and hence the physiologic breakdown of blood clots (56). It has been reported that a depletion of human WRN protein with small interference RNA molecules leads to an overexpression of PAI-1 in human fibroblasts (57). Similarly, we have found a significant increase in serum PAI-1 level in WrnΔhel/Δhel mice (29). Moreover, the serum concentration of ascorbate seems to regulate the expression of PAI-1. Indeed, we have reported that Gulo−/− mice without ascorbate treatment exhibit high serum levels of PAI-1 that is decreased upon ascorbate treatment (21). In the present study, we also observed an increase in serum PAI-1 in Gulo−/− mice treated with 0.01% ascorbate compared with WT mice. More important, we observed an additive effect of the Wrn and Gulo mutations as PAI-1 levels were even higher in WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate than in WT mice. This phenotype was completely reversed by 0.4% ascorbate treatment. PAI-1 is present in increased levels in various disease states such as cardiovascular diseases (during the development of vessel wall damage) (58) and metabolic syndrome (59). In inflammatory conditions, PAI-1 is secreted by endothelial cells and appears to play a significant role in the progression to fibrosis (60, 61). We found in other work that we can detect an increase in this cardiovascular risk factor in young WrnΔhel/Δhel mice before they exhibit cardiac fibrosis and valvular aortic stenosis (13, 37).
Oxidative stress in the ER fraction of WrnΔhel/Δhel/Gulo−/− mice
Oxidative stress has been implicated in a wide variety of degenerative processes and age-related diseases (62, 63). WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate showed a higher level of ROS in comparison with age-matched WT littermates (Fig. 8A). This result is similar to what we reported for the total liver of Gulo−/− mice treated with 0.01% ascorbate in drinking water (21). Moreover, we observed a significant increase in the ratio of Met-SO:Met in the serum of both WrnΔhel/Δhel/Gulo−/− and Gulo−/− mice treated with 0.01% ascorbate compared with that in WT mice (Fig. 7A). The ratio of serum Met-SO:Met is known increase in several pathologic and inflammatory processes (28, 64). ROS levels or serum Met-SO:Met ratio did not increase in total liver extract of WrnΔhel/Δhel mice compared with age-matched WT mice, as reported (29). A priori, these results indicated that the levels of oxidative stress in serum and total liver extracts of WrnΔhel/Δhel/Gulo−/− and Gulo−/− mice treated with 0.01% ascorbate would be similar. However, when we examined the mutation rate of mitochondrial DNA in our different mouse cohorts at 4 mo of age, oxidative stress was significantly higher only in WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate (Table 1). This mitochondrial DNA mutation rate was decreased by 0.4% ascorbate treatment to the WT level, as were the ROS levels in total liver. Ascorbate is believed to be used predominantly as an antioxidant in mitochondria (65), suggesting a more severe shortage of ascorbate in the mitochondria of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate compared with Gulo−/− mice treated with 0.01% ascorbate.
Another significant difference between WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate and all the other mouse genotypes was found in the ER stress response. ROS levels in ER enriched fractions from the liver of 0.01% ascorbate treated WrnΔhel/Δhel/Gulo−/− mice was approximately twice the levels found in age-matched WT mice (Fig. 8B). We have reported that WrnΔhel/Δhel mice exhibit a 20% increase in ROS levels in the liver ER enriched fractions compared with age-matched WT mice (29). The ER is the main site for the synthesis and folding of around one-third of the total proteome of a cell (66). Proper folding of nascent protein in the ER requires an oxidant environment. However, too much ROS in the ER may cause irreversible modifications to nascent proteins that are unable to fold correctly (67). The thiol-SH side chain of cysteine is susceptible to reaction with ROS, giving rise to a range of oxidative post-translational modifications that can affect the formation of disulfide bonds within or between peptide chains (64, 68). With the use of the Ellman reagent, we recorded a significant increase in total thiol content in the liver lysates of WrnΔhel/Δhel/Gulo−/− mice treated with 0.4% ascorbate compared with WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate.
The 3 branches of the ER response pathways were significantly activated in WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate compared with age-matched WT mice (Fig. 9). More specifically, cleaved ATF6 signal was increased, and total protein levels of PERK and IRE1α were increased. This finding is different from what we had reported for WrnΔhel/Δhel and Gulo−/− mice. We had observed a significant increase in total IRE1α protein levels only in WrnΔhel/Δhel mice compared with age-matched WT mice (11) and a significant increase in total PERK protein level in Gulo−/− mice treated with 0.01% ascorbate (21). These results indicate that oxidative stress is more severe in the hepatic ER of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate than in WrnΔhel/Δhel or Gulo−/− mice so treated.
Unfolded or damaged proteins can be targeted to the proteasome for degradation via ubiquitylation (69). HERPUD1 is an ER membrane protein that facilitates the degradation of ubiquitylated proteins through ERAD (35, 70). Total ubiquitylated proteins and HERPUD1 expression levels increased significantly in WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate compared with age-matched WT mice. Upon 0.4% ascorbate treatment, HERPUD1 and total ubiquitylated protein levels normalized to the WT level. In parallel, the total PERK protein level was reversed to WT level upon 0.4% ascorbate treatment in the liver of WrnΔhel/Δhel/Gulo−/− mice. Notably, HERPUD1 expression is regulated by the PERK-dependent pathway during ER stress (71). In contrast, the levels of cleaved ATF6 and IRE1α proteins were still significantly higher in 0.4% ascorbate treated WrnΔhel/Δhel/Gulo−/− mice than WT mice (Fig. 9). Moreover, CHOP protein level increased significantly in 0.4% ascorbate treated WrnΔhel/Δhel/Gulo−/− mice. The CHOP response to the 0.4% ascorbate treatment in the liver of WrnΔhel/Δhel/Gulo−/− mice is intriguing as CHOP is known to suppress adipogenesis and limits expansion of fat mass in vivo in mice potentially allowing a longer survival time (72).
Like the WrnΔhel/Δhel mice, WrnΔhel/Δhel/Gulo−/− mice synthesize a stable Wrn mutant protein that is also found in the ER enriched fractions of liver tissues (Fig. 9) (11). Addition of 0.4% ascorbate to the drinking water did not decrease this mutant WrnΔhel protein that can potentially induce chronic ER stress in WrnΔhel/Δhel/Gulo−/− mice. The higher levels of cleaved ATF6 and IRE1α proteins in WrnΔhel/Δhel/Gulo−/− mice, even in the presence of 0.4% ascorbate in the drinking water, may represent a continuous chronic ER stress response. The constant high levels of cleaved ATF6 and IRE1α may allow WrnΔhel/Δhel/Gulo−/− mice to cope with this continuous stress. Similarly, WrnΔhel/Δhel/Gulo−/− mice exhibit a significant increased PDI protein levels in the ER enriched fractions during both 0.01 and 0.4% treatments. PDI plays a pivotal role in proper protein folding by disulfide bond formation (73, 74). Intracellular levels of ascorbate and its reduced form dehydroascorbate (DHA) are known to modulate the reduced status of the catalytic dithiol center of PDI, and hence its enzymatic activity (75). Ascorbate is an excellent electron donor and leads to DHA upon oxidation (65). However, the half-life of DHA is very short in the ER and can be reduced by PDI, oxidizing the active central dithiols of this enzyme. Oxidized PDI, in return, reacts with reduced substrate proteins yielding protein disulfides and finally a decrease in proper protein folding. Catalytically active PDI is regenerated in this last step (65). Alternatively, DHA can rapidly react with dithiols in unfolded or partially folded proteins in a PDI-independent manner (65). Thus, the greater availability of ascorbate in drinking water would ultimately improve PDI reactions and proper disulfide bonding of nascent peptides during protein synthesis in WrnΔhel/Δhel/Gulo−/− mice. Overall, the elevated mitochondrial DNA mutation rate, the increased ROS in the ER enriched fraction, the major chronic ER stress, the activation of the ERAD pathway, and the very low level of serum ascorbate in WrnΔhel/Δhel/Gulo−/− mice given only 0.01% ascorbate in the drinking water are reminiscent of ascorbate compartmentation disease in these mice (65, 76). In our WrnΔhel/Δhel/Gulo−/− mouse model treated with 0.01% ascorbate, there is not enough ascorbate for proper function of the ER compartment, leading to a chronic ER stress response.
CONCLUSIONS
An important step in future studies is to find the optimal ascorbate doses that have the maximum positive impact on health. Unfortunately, high doses of ascorbate can have deleterious side effects. For example, ascorbate is continuously catabolized by oxidation to DHA. Major alterations in the ascorbate/DHA balance toward a more pro-oxidant state in different cellular organelles including the ER may negatively affect specific biochemical reactions and the redox status of the cells (65, 76). In addition, oxalate is a major end product of ascorbate in humans that can cause accumulation of calcium oxalate stones and nephrocalcinosis (76). Ascorbate is also a cofactor for many enzymes in the ER that are important for the synthesis of proteins of the extracellular matrix, and it has an impact on the proliferation, maintenance, and differentiation of stem cells (76). A low level of ascorbate may lead to developmental defects in different tissues, whereas too much could push preneoplastic cells toward more aggressive metastatic cells (76). Thus, appropriate in vivo models are necessary to gain important insights into the molecular processes modulated by different supplementation levels of ascorbate. In the present study, we generated a unique mouse model for WS that, as in humans, relies entirely on a dietary source of ascorbate to survive. These WrnΔhel/Δhel/Gulo−/− mice exhibited a severe reduction in their lifespan, lower body weight, sterility, and osteopenia when treated with a suboptimal dose of ascorbate. The metabolic and cytokine profiles of WrnΔhel/Δhel/Gulo−/− mice treated with 0.01% ascorbate were unique and very different from those of WT, WrnΔhel/Δhel, and Gulo−/− mice. Even the serum ascorbate level was significantly reduced compared with Gulo−/− mice treated with 0.01% ascorbate in drinking water. Although the phenotypes of WrnΔhel/Δhel/Gulo−/− mice were greatly improved upon 0.4% ascorbate treatment, the metabolic profile was different from age-matched WrnΔhel/Δhel and WT mice. Finally, the severe reduction of serum ascorbate in WrnΔhel/Δhel/Gulo−/− mice revealed a chronic oxidative stress in the ER and a permanent activation of all branches of the ER stress response pathways. In addition, markers associated with the ubiquitin-proteasome–dependent ER-associated degradation pathway were increased. Augmenting the dose of ascorbate to obtain optimal serum vitamin C concentration reversed the activation of this pathway to WT levels, indicating that the pathway is a potential therapeutic target in WS.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by funding from the Canadian Institutes of Health Research (to M.L. and A.M.), and by U.S. National Institutes of Health (NIH) National Institute on Aging Grant R01AG028873 (to R.J.P.). L.A. is a scholar from the Fondation du Centre Hospitalier Universitaire de Québec. The authors declare no conflicts of interest.
Glossary
| ATF | cAMP-dependent transcription factor |
| CHOP | C/EBP-homologous protein |
| DCFA | 2′-7′ dichlorofluorescein diacetate |
| DHA | dehydroascorbate |
| DTNB | 5,5′-dithio-bis-(2-nitrobenzoic acid) |
| eIF2α | eukaryotic translation factor 2-α |
| ER | endoplasmic reticulum |
| ERAD | ER-associated degradation |
| G-CSF | granulocyte colony-stimulating factor |
| Gulo | gulonolactone oxidase gene |
| H&E | hematoxylin and eosin |
| HERPUD | homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member |
| HSC | heat shock cognate |
| IRE | inositol-requiring enzyme |
| LC | liquid chromatography |
| M-CSF | macrophage–colony-stimulating factor |
| Met-SO | methionine-sulfoxide |
| MIP | macrophage inflammatory protein |
| MS | mass spectrometry |
| PAI | plasminogen activator inhibitor |
| PC | phosphatidylcholine |
| PCA | principal component analysis |
| PC aa | PC diacyl |
| PC ae | PC acyl-alkyl |
| PDI | protein disulfide isomerase |
| PERK | eukaryotic translation initiation factor 2-α kinase-3 |
| ROI | region of interest |
| ROS | reactive oxygen species |
| Wrn | Werner syndrome RecQ-like helicase gene |
| WS | Werner syndrome |
| WT | wild type |
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. Aumailley, E. R. Paquet, R. J. Pignolo, A. Marette, and M. Lebel designed the research; L. Aumailley, M. J. Dubois, T. A. Brennan, C. Garand, and E. R. Paquet performed the research; E. R. Paquet, R. J. Pignolo, A. Marette, and M. Lebel contributed new reagents and analytic tools; L. Aumailley, M. J. Dubois, T. A. Brennan, E. R. Paquet, R. J. Pignolo, and M. Lebel analyzed the data; L. Aumailley and M. Lebel wrote the paper; and L. Aumailley, M. J. Dubois, T. A. Brennan, E. R. Paquet, R. J. Pignolo, A. Marette, and M. Lebel contributed intellectually to the paper.