- Journal List
- HHS Author Manuscripts
- PMC4020596

mTOR Regulation and Therapeutic Rejuvenation of Aging Hematopoietic Stem Cells
Chong Chen
1Department of Surgery, University of Michigan, School of Medicine and Comprehensive Cancer Center. Ann Arbor, MI48109
Yu Liu
1Department of Surgery, University of Michigan, School of Medicine and Comprehensive Cancer Center. Ann Arbor, MI48109
Yang Liu
1Department of Surgery, University of Michigan, School of Medicine and Comprehensive Cancer Center. Ann Arbor, MI48109
2Department of Internal Medicine, University of Michigan, School of Medicine and Comprehensive Cancer Center. Ann Arbor, MI48109
Pan Zheng
1Department of Surgery, University of Michigan, School of Medicine and Comprehensive Cancer Center. Ann Arbor, MI48109
3Department of Pathology, University of Michigan, School of Medicine and Comprehensive Cancer Center. Ann Arbor, MI48109
Associated Data
Abstract
Age-related declines in hematopoietic stem cell (HSC) function may contribute to anemia, poor response to vaccination, and tumorigenesis. Here, we show that mammalian target of rapamycin (mTOR) activity is increased in HSCs from old mice compared to those from young mice. mTOR activation through conditional deletion of Tsc1 in the HSC of young mice mimicked the phenotype of HSC from aged mice in various ways. These included increased abundance of the mRNA encoding the CDK inhibitors p16Ink4a, p19Arf, and p21Cip1, a relative decrease in lymphopoiesis, and impaired capacity to reconstitute the hematopoietic system. In the old mice, rapamycin increased the life span, restored the self renewal and hematopoiesis of HSC, and enabled effective vaccination against a lethal challenge with influenza virus. Together, our data implicate mTOR signaling in HSC aging and demonstrate the potential of mTOR inhibitors for restoring hematopoiesis in the elderly.
Introduction
Hematopoietic stem cells (HSCs) show decreased function with age; these functional deficits include reduced self-renewal, hematopoiesis, and differentiation into lymphocytes (1–5). The ensuing decrease in lymphopoiesis likely contributes to the weakened adaptive immune response characteristic of the elderly (5). Both cell-intrinsic and extrinsic mechanisms may contribute to these age-related changed in HSC function (1, 6–9); however, the underlying molecular pathways have not been elucidated.
The mammalian target of rapamycin (mTOR) pathway integrates multiple signals from nutrients, growth factors, and oxygen to regulate cell growth, proliferation, and survival (10–12). Here, we describe an increase in mTOR signaling in HSC from aged mice and show that inhibition of mTOR signaling with rapamycin restores HSC function and enhances the immune response to influenza virus in old mice. Moreover, mTOR signaling has also been shown to regulate the longevity of yeast (13), Caenorhabditis elegans (14), and Drosophila (15). The data herein and a recent report (16) indicate increased life-span of rapamycin-treated mice. Thus, mTOR activation may represent a conserved mechanism for aging in yeast, C. elegans, Drosophila and mammal.
Results
Dysregulation of the mTOR Pathway in HSC from aged mice
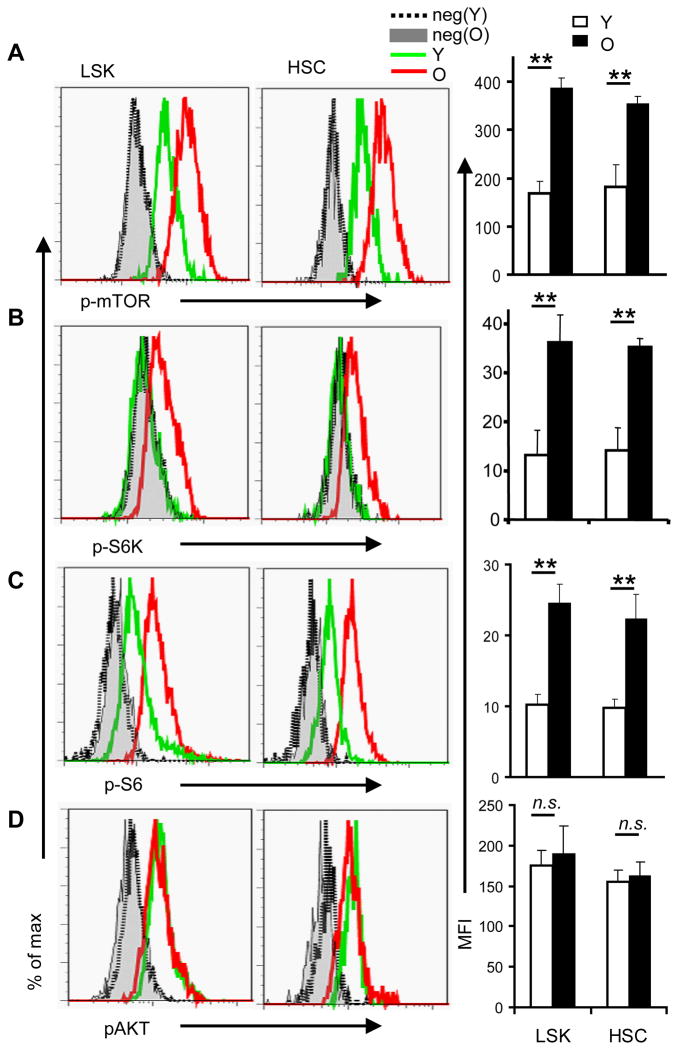
We isolated bone marrow cells from young (2 month old) and old (26 month old) C57BL/6 mice and analyzed them for surface markers to identify HSCs, and for intracellular staining of phosphorylated mTOR (p-mTOR). Using flow cytometry, we found that the amount of p-mTOR was significantly increased in both HSC-enriched Lin− Sca-1+c-Kit+ (LSK) and the Flk2− lin− Sca-1+c-kit+ CD150+CD48− CD34− (FLSKCD150/48/34) HSCs from old mice compared to that in HSCs (Fig. 1A) from young mice. Consistent with the increase in phosphorylated mTOR, the abundance of the phosphorylated form of the mTOR complex 1 (mTORC1) substrate S6K and of the S6K substrate S6 was significantly increased in HSCs from old mice compared to that in HSCs from young mice (Fig. 1B–C). These data indicate that the overall activity of mTOR in HSCs from old mice is greater than that in HSCs from young ones. To see whether this increase in mTOR phosphorylation was secondary to increased activity in the phosphoinositide 3-kinase (PI3K)-AKT signaling pathway, we evaluated AKT activation by measuring the abundance of AKT phosphorylated on Ser residue 473 (pAKT) by flow cytometry. We found that the amount of pAKT in HSC from young and old mice was indistinguishable (Fig. 1D).
mTOR activity in young and aged HSCs. Fresh BM cells were isolated from either 2-month (young) or 26-month (old) old wildtype mice and stained with antibodies specific for surface markers to identify the Flk2− lin− Sca-1+c-kit+ CD150+CD48− CD34− (FLSKCD150/48/34) HSC or the HSC-enriched lin− Sca-1+c-kit+ (LSK) cells, followed by intracellular staining with antibodies against p-mTOR (A), p-S6K (B), p-S6 (C) and p-AKT (D). The left panels show the histograms of HSC that are representative of 5 independent experiments each involving one mouse per group. Gray lines are negative controls (secondary antibodies only for a, b and d, isotype control for c), with filled grey depicting cells from old mice, while dotted lines depicting cells from young mice; green lines depict profiles of stained BM cells from young mice and red lines depict those of BM cells from old mice. The maximum depicts the point with highest cell count. The mean fluorescence intensity (MFI) ± SD of all experiments are shown in the right panels. *, p<0.05; **, p<0.01. n.s., not significant.
Tsc1 deletion is sufficient to induce premature aging of HSC
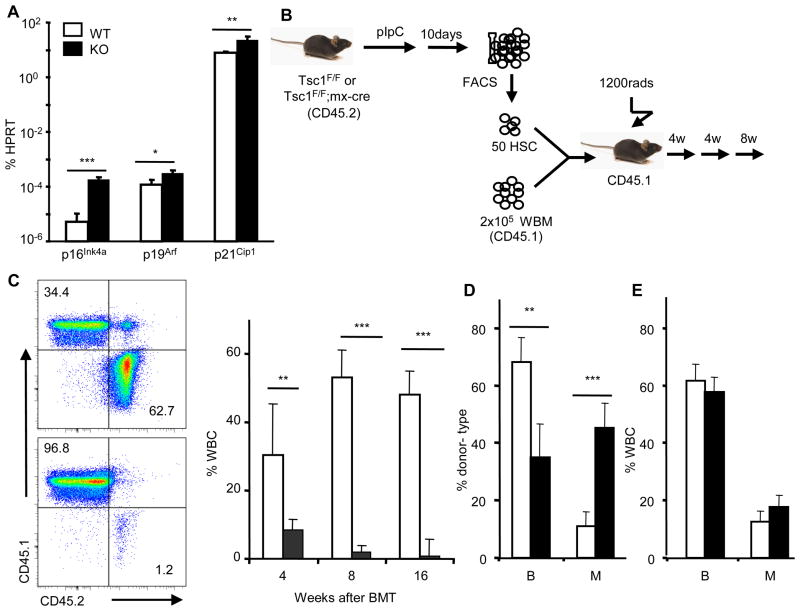
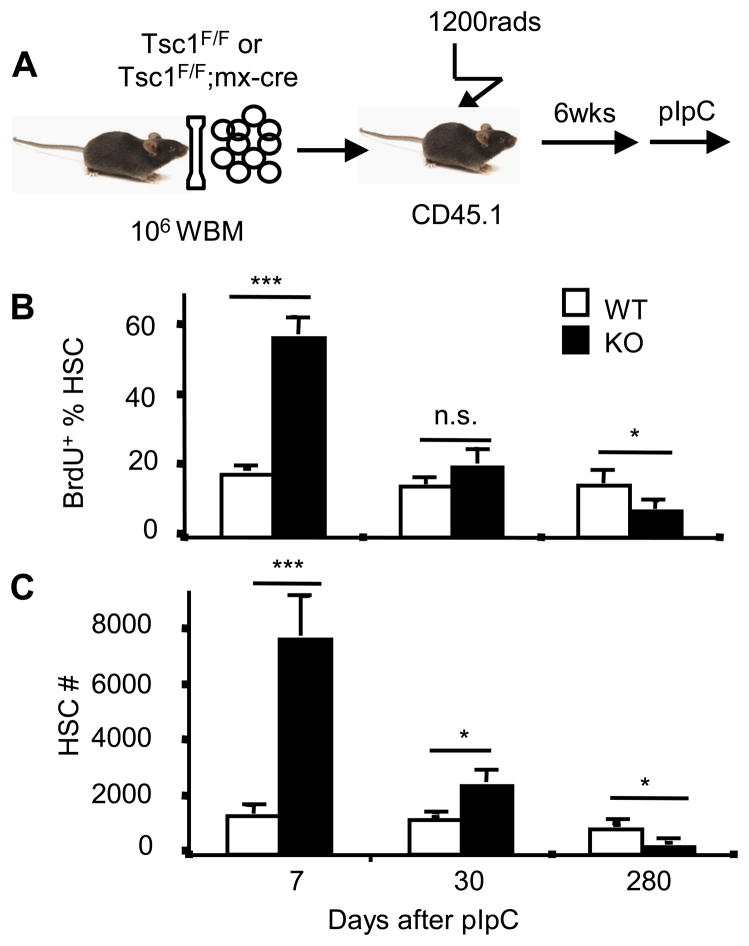
To determine whether increased mTOR signaling could explain the functional deficits of HSC from old mice, we deleted the Tsc1 gene in the HSCs of young adult mice. Deletion of Tsc1, which encodes tuberous sclerosis complex (TSC) protein 1, leads to constitutive activation of mTOR in HSCs (17). The abundance of the mRNAs encoding p16Ink4a, p19Arf, and p21Cip1 were all significantly increased in Tsc1−/− HSC (Fig. 2A). To test the effect of mTOR signaling on the hematopoiesis capacity of HSCs, we used the Mx1-Cre transgene for conditional deletion of the Tsc1flox/flox gene in the HSC following polyinosine-polycytidylic acid (pIpC) treatments. We transplanted, into lethally irradiated B6Ly5.2 recipients, 2×105 recipient-type (B6Ly5.1) bone marrow cells in conjunction with 50 HSCs (FLSKCD150/48/34) isolated 10 days after ppIpC treatment from either Tsc1flox/flox; Mx1-cre+ or Tsc1flox/flox; Mx1-cre− mice (Fig. 2B). The function of HSC was indicated by hematopoiesis from the donor-type HSC in the recipients. At 4 weeks after transplantation with cells from wild-type donors, 30% of leukocytes in the peripheral blood of the recipients were derived from donor HSCs. The ratio of leukocytes derived from wild-type donor HSCs to recipient-type leukocytes increased to around 50% at 8 weeks. In contrast, Tsc1-deficient HSCs gave rise to only about 8% of the leukocytes present at 4 weeks and by 16 weeks their contribution was barely detectable (Fig. 2C). Furthermore, leukocytes derived from the Tsc1-deficient HSCs showed markedly reduced ratios of B lymphocytes (B220+) to myeloid cells (Mac-1+ or Ter119+ or both) (Fig. 2D). The reduction is HSC-intrinsic because hematopoiesis from host-derived cells was unaffected (Fig. 2E). Although Tsc1 deletion causes an immediate increase in HSC proliferation (17), self-renewal of the transplanted Tsc1−/− HSC decreased over time (Fig. 3). The reduced BrdU incorporation of the Tsc1−/− HSC at 280 days after transplantation recapitulates a feature of HSC from old mice(18) (Also see Fig. 5 below).
Tsc1 deficiency and premature aging of HSC from young mice. A. six week old Tsc1flox/flox (WT) and Tsc1flox/flox; Mx1-cre+ (KO) mice were treated 7 times with pIpC every other day for 14 days and sacrificed 10 days after the last treatment. The abundance of p16Ink4a, p19Arf, and p21Cip1 mRNAs in FACS-sorted HSCs was measured by real-time PCR and normalized to hypoxanthine-guanine phosphoribosyltransferase (Hprt). n=5. B–E. 50 HSCs were mixed with 2×105 recipient-type total BM cells (WBM) and transplanted into lethally irradiated B6Ly5.2 recipients. The donor and recipient-type cells were identified by congenic CD45 markers. B. Diagram of the experimental design for C–D. C. Hematopoiesis by WT and Tsc1−/− HSCs. Left shown the representative dot plots of the recipient peripheral blood at 16-weeks after transplantation with WT (top) or Tsc1−/− cells (bottom). Data on the right summarize two independent experiments with a total of 10 recipients for each group. D. The percent of the donor HSC-derived and B220+ (B) and Mac-1+ (M) cells in the recipient peripheral blood at 4 weeks after transplantation. E. The percentage of B and M cells in total white blood cells.
Targeted mutation of Tsc1 in bone marrow cells resulted in transient increase but long-term reduction in HSC self-renewal. A. The diagram of experimental design. B. BrdU incorporation of HSCs from WT- or KO- donors after 24h labeling. n=4. C. HSC numbers derived from WT- or KO- donors in the recipients after pIpC treatment. n=4.
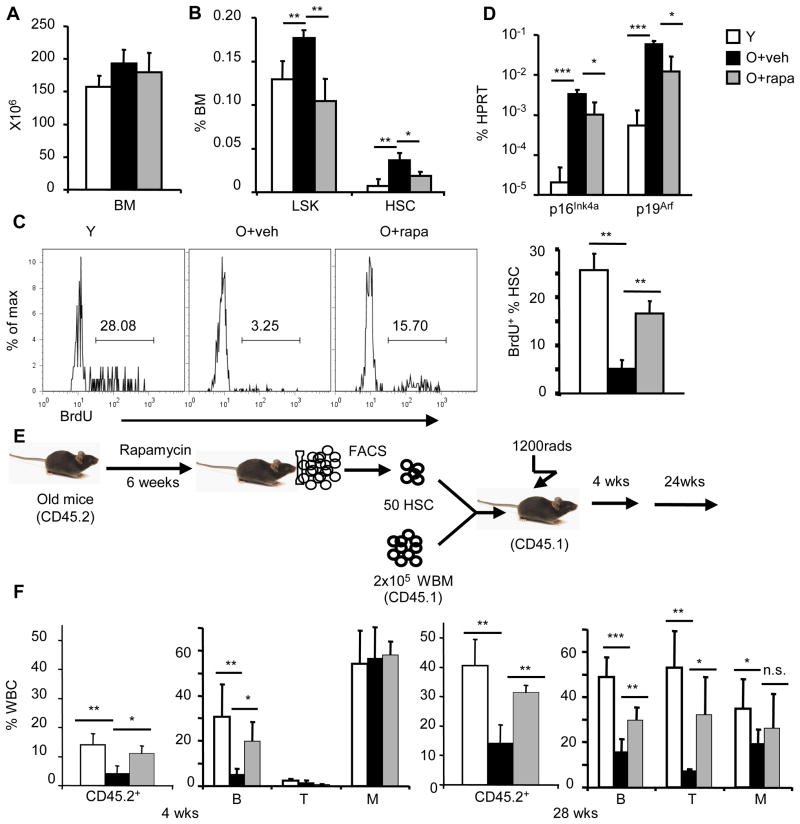
Rapamycin rejuvenates HSC from old mice. A. Bone marrow cellularity of tibia and femurs of young (Y, 2 months of age) mice, or age-matched, 22–24 months old mice treated with either vehicle (O-veh) or old mice treated with rapamycin (O-rapa). n=5. B. The percentage of LSK and FLSKCD150/48/34 HSC populations in bone marrow of Y, O-veh and O-rapa mice. n=5. C. BrdU incorporation. Left panels show representative profiles of BrdU incorporation in HSCs, and right panels show Mean ± SD of BrdU+ cells (n=4). D. HSCs were sorted by FACS and p16Ink4a and p19Arf mRNA abundance was measured by real-time PCR and normalized to Hprt abundance; values shown are mean ± SD (n=3). E. Diagram of experiments. F. Hematopoietic function of HSC as measured by CD45 congenic markers on leukocytes of recipient peripheral blood at 4 weeks and 28 weeks after transplantation. The left panels show the % of donor-type cells among leukocytes (CD45+) and right panels show the reconstitution rates of B220+ (B), CD3+ (T) and Mac-1+ (M) lineages. Data shown were from three independent experiments with 15 recipients per group.
Rapamycin restores HSC function in the old mice
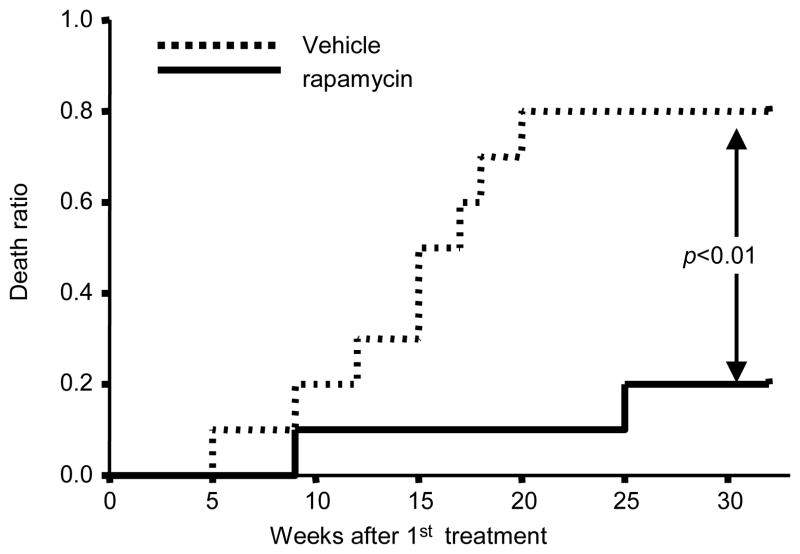
Because mTOR signaling is increased in HSCs from aged mice, we wondered whether inhibition of mTOR could reverse the functional deficits characteristic of HSCs from old mice. We treated old mice (22 months old) with either vehicle or the mTOR inhibitor rapamycin at a dose of 4mg/kg, every other day for 6 weeks and monitored their life span. We found that rapamycin treatment significantly extended the life span of aged mice (Fig. 4). Moreover, without affecting the overall cellularity of bone marrow (Fig. 5A), rapamycin treatment reduced the percentage and the absolute number of HSCs (Fig. 5B). These data suggest that the rapamycin-treated HSC from old mice might have enhanced regenerative capacity. To test this notion, we pulsed young, vehicle-treated old, or rapamycin-treated old mice with 5-Bromo-2′-deoxyuridine (BrdU) for 52 hours starting 2 days after the last rapamycin treatment. We determined the proportion of BrdU+ cells among HSC by flow cytometry. Only 5% of the HSCs from vehicle-treated old mice were BrdU+; however, pretreatment with rapamycin caused a significant increase in the proportion of BrdU positive HSCs (16%) (Fig. 5C). Rapamycin pretreatment also decreased the expression of HSC p16Ink4a and p19Arf (Fig. 5D), which have been reported to contribute to aging of both HSC and neuronal stem cells (18, 19). To test the functional effects of rapamycin treatment on HSCs from old mice more directly, we transplanted 50 HSCs from old mice treated with vehicle or rapamycin into lethally-irradiated young recipients, in each case in conjunction with 2×105 recipient-type bone marrow cells. The efficacy of hematopoietic reconstitution by transplanted HSCs was assessed by the percentage of donor-type peripheral blood cells in the recipients (Fig. 5E). Consistent with previous reports, hematopoietic reconstitution by HSCs from old mice was decreased compared to that by HSCs from young mice (1, 20). However, both 4 weeks and 28 weeks after transplant, the fraction of circulating leukocytes derived from rapamycin-treated HSCs from old mice was significantly increased compared to that derived from vehicle-treated HSCs (Fig. 5E). Thus, inhibition of mTOR activity by rapamycin enhances the in vivo regenerative capacity of HSCs from old mice and this enhancement is cell-autonomous.
Effect of rapamycin on life span of old mice. Age-matched 22–24 month old B6 mice were treated with vehicle or 4mg/kg bodyweight rapamycin by intraperitoneal injection every other day for 6 weeks. Survival of mice following the first rapamycin treatment were monitored on daily basis. Death ratio refers to fraction of mice that are either dead or moribund, n=10.
Short-term rapamycin treatment boosts immunity of the old mice
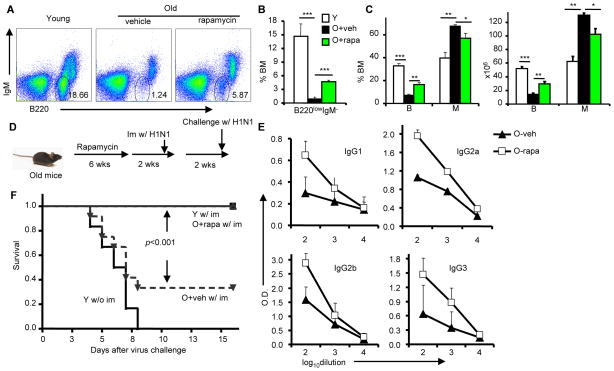
Old mice have impaired B cell generation; in particular, they show decreased numbers of pre-B cells (21, 22). We noted enhanced production of B lymphocytes and decreased myelogenesis in rapamycin-treated old mice (Fig. 6A–C). Remarkably, rapamycin treatment doubled the percentage and the number of B220+ B cells in the bone marrow (Fig. 6C). Rapamycin treatment also quadrupled the percentage of B220low IgM− pre-B cells, which was very low in the vehicle-treated group (Fig. 6A–B).
Vaccination-induced protection in old mice after vehicle or rapamycin treatment. a–c, 22–24 month old B6 mice were treated with vehicle or 4mg/kg body weight rapamycin by intraperitoneal injection every other day for 6 weeks. A. Representative FACS files of BM cells from young (Y) and old mice treated with either vehicle (O-veh) or rapamycin (O-rapa). n=5. B. The percentage of immature B progenitor cells (B220lowIgM−) in whole bone marrow, gated as in (A). C. The percentage (left panel) and absolute number (right panel) of B220+ B lineage cells and Mac-1+ myeloid lineage cells in whole bone marrow. D–F, 26–28 month old B6 mice were treated with vehicle or 4mg/kg body weight rapamycin by intraperitoneal injection every other day for 6 weeks. Two weeks later, young and vehicle- or rapamycin-treated old mice were immunized with 200 HAU of A/PR/8/34 virus by i.p. injection. D. The diagram of experimental design for e–f. E. 10 days after immunization, influenza-specific antibodies in the plasma of peripheral blood were measured by ELISA. F. Two-weeks after immunization, mice were challenged with 400 HAU of A/PR/8/34 virus by intranasal delivery. Data shown are Kaplan-Meier survival analysis. n=12. The P value is derived from a log-rank test.
Because rapamycin treatment enhanced the generation of B cells in old mice, we hypothesized that it might also improve their immune response. To test this idea, we treated 26-month old mice for 6 weeks with either vehicle or rapamycin. After pausing for 2 weeks to avoid the possible suppression of the ongoing immune response by rapamycin (23), we immunized the mice with 200 hemagglutinin units (HAU) of influenza virus by intraperitoneal (i.p.) injection (Fig. 6D). Naïve and immunized young mice were used as controls. Ten days after immunization, we assessed the immune response by measuring by the abundance of influenza virus-specific antibodies in the peripheral blood. We focused on IgG antibodies because they are more effective against influenza virus than IgM and IgA (24). As shown in Fig. 6E, pretreatment with rapamycin increased the amounts of antigen-specific IgG by approximately 10 times for all isotypes of virus-specific IgG in the old mice. Rapamycin treatment did not increase the percentage of T cells (fig. S1). Nevertheless, the % of influenza CD8 T cells was higher in old mice than that in the young mice (fig. S2), regardless of rapamycin treatment. To test whether rapamycin pretreatment affects the response to vaccination, we challenged the vaccinated mice with the same strain of influenza virus at a dose that is lethal to unimmunized young mice. As shown in Fig. 6F, 9/12 (75%) vehicle-treated old mice succumbed to infection even after immunization, whereas all mice that had been pre-treated with rapamycin were protected by the vaccination.
Discussion
Together, our data show that the activity of the mTOR pathway is increased in HSCs from aged mice and that increasing mTOR signaling is sufficient to cause premature aging of HSCs in young mice. These data demonstrate a fundamental role for mTOR signaling in HSC aging and thus reveal a functional conservation in mechanisms of aging from yeast (13), C. elegans (14) and Drosophila (15) to mammals. More importantly, pharmaceutical inhibition of mTOR improved the regenerative capacity of HSCs from aged mice, showing that the functional capacity of these HSCs can be restored. We also found that mTOR inhibition reinitiated B cell development and enabled aged mice to mount a robust antibody response capable of protecting them against lethal influenza infection. Because aging impairs B cell development, the elderly have more memory B cells relative to naïve B cells than do the young mice, and this imbalance contributes to impaired immune responses in elderly individuals (25, 26). The elderly mount a poorer immune response to influenza vaccination (27), and this response may contribute to the significant morbidity and mortality caused by influenza virus in the elderly, even among vaccinated individuals (28). Our data suggest that rapamycin, or possibly other inhibitors of mTOR, can be used to rejuvenate HSC for better immune protection. We showed that a short-term rapamycin treatment can have a long-lasting effect on the HSC function, thus avoiding potential toxicity associated with chronic treatment. The shorter therapeutic duration may have resulted in a more significant increase in mouse life span than that achieved by the chronic treatment (16). It is also of note that although rapamycin has been used as immune suppressants in transplantation (23), treatment of rapamycin during infection can paradoxically induce a more effective memory T cell response (29).
Materials and Methods
Mice
Old C57BL/6 wildtype mice were obtained from National Institute of Aging. Tsc1fl/fl mice (30) were provided by D.J. Kwiatkowski (Brigham and Women’s Hospital, Boston, MA) and were backcrossed for at least six generations onto the C57BL/6 background. Mx1-cre mice were purchased from the Jackson Laboratory. Recipients in reconstitution assays were 8-week old B6ly5.2 mice from National Cancer Institute. All the mice were kept in the Unit of Laboratory Animal Facility (ULAM) at University of Michigan, Ann Arbor. And all procedures involving experimental animals were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
pIpC and rapamycin treatment
pIpC (Sigma-Aldrich) was resuspended in 1xPBS at 1 mg/ml. Mice received 15 mg/kg of pIpC every other day seven times by i.p. injection. Rapamycin (LC Laboratories) was reconstituted in ethanol at 10 mg/ml and then diluted in 5% Tween-80 (Sigma-Aldrich) and 5% PEG-400 (Hampton Research). Mice received 4 mg/kg rapamycin by i.p. injection every other day for 6 weeks (17).
Transplantation assays
8-wk-old congenic recipient mice were lethally irradiated with a Cs-137 x-ray source delivering 97 rads per min. A combined 1,150 rads was delivered in two installments 4 hours apart. Given numbers of donor BM cells were transplanted into recipients through the retro-orbital venous sinus within 24h of irradiation. For HSC transplant, FLSKCD150/48/34 HSCs were sorted by FACSAria (BD Biosciences), mixed with indicated number of recipient type BM cells. Reconstitutions were measured by flow cytometry of peripheral blood from the recipient tail vein at the time points indicated.
Flow cytometry
BM cells were flushed out from the long bones (tibiae and femurs) by a 25-gauge needle with 1xHanks Balanced Saline Solution without calcium or magnesium (Invitrogen), supplemented with 2% heat-inactivated fetal bovine serum. Peripheral blood was obtained from the tail veins of recipients at the time points indicated in the figures, and RBC were lysed by ammonium chloride-potassium bicarbonate buffer before staining. For flow cytometry and purification of HSCs, the immunophenotype FLSKCD150/48/34 were used for long-term (LT)-HSCs as indicated. Lineage markers included B220 (B cells), CD3 (T cells), Gr-1 (granulocyte), Mac-1 (myeloid cells), and Ter119 (erythrocytes). CD150 was from BioLegend, Inc. All other antibodies were obtained from BD Bioscience. For intracellular staining, cells were first stained with the indicated surface markers and then fixed with Fix buffer (BD Bioscience) for 2 hours at 4°C, followed by incubation with BD cytoperm+ buffer for 10 minutes at room temperature and re-fix for 10 minutes. p-mTOR (Ser2448), p-S6K (Thr389), and AKT (Ser473) antibodies (Cell Signaling Technology) were diluted at 1:100 and AlexaFluor 488 conjugated pS6 (pSer235/236) antibodies (Cell Signaling Technology) were diluted at 1:10, and incubated overnight at 4°C. FITC conjugated secondary antibodies (Jackson Immunoresearch) were diluted at 1:100 and incubated for 2 hours at 4°C. Flow cytometry analysis was performed on an LSR II (BD Biosciences).
BrdU incorporation
BrdU (Sigma-Aldrich) was injected i.p. into adult mice at 100 mg/kg bodyweight. Mice were then given 1 mg/ml BrdU water for 24 (Fig. 3) or 52 (Fig. 5) hours as indicated before sacrificed for analysis. BrdU staining kit (BD Bioscience) was used according to the producer’s manual.
Real-time PCR
HSCs were purified by FACS sorting and RNA was isolated with Trizol (Invitrogen) and further purified by RNeasy kit (Invitrogen). cDNA was made from purified RNA with Superscript III (Inivitrogen). Real-time PCR was performed on 7500 Real Time PCR system (Applied Biosystems).
Immunization and ELISA
Mice were immunized with 200 HAU Influenza A/PR/8/34(H1N1) (Charles River Laboratories, Wilmington, Massachusetts) by i.p. injection. Ten days after immunization, peripheral blood was collected and plasma was used for enzyme-linked immunosorbent assay (ELISA). To test the relative levels of influenza-specific antibodies, 96-well ELISA plates were coated with 0.4 μg/ml inactivated and purified influenza A/PR/8/34(H1N1) virus (Charles River Laboratories, Wilmington, Massachusetts). Plasma was serially diluted by 1:100, 1:1000 and 1:10000. Secondary antibodies were HRP-conjugated goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 (Southern Biotechnology Associates, Birmingham, AL.), respectively, diluted at 1:1000. Color was developed by BD OptEIA (BD Bioscience) for 15 min for IgG2a and IgG2b, 30 min for IgG1 and IgG3 and terminated by acid. Two weeks after immunization, mice were challenged with 400 HAU H1N1 influenza virus through intranasal delivery.
Statistics
The function of HSCs in the rescue of lethal irradiation was compared by a Kaplan-Meier survival analysis, and the p-value of the log-rank tests are provided either in the figures or in the figure legends. Student’s t tests were used for all other analyses (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Acknowledgments
We thank Dr. Richard Miller for critical reading of the manuscript. This study is supported by grants from American Cancer Society (RSG-06-072-01-TBE), the US Department of Defense (W81XWH-07-1-0169 and W81XWH-08-1-0036) and National Institutes of Health (CA112001 and AG024824). The aged animals were provided through the Pepper Center’s Core Facility for Aged Rodents. The authors have no financial conflict of interest.