- Journal List
- J Appl Physiol (1985)
- PMC8409925

Skeletal muscle-specific calpastatin overexpression mitigates muscle weakness in aging and extends life span
 1
,
2
,
3
Lin Wang,
1
Yuan Wen,
2
,
3
Leigh Ann P. Callahan,
1
,
3
and Gerald S. Supinski
1
,
3
1
,
2
,
3
Lin Wang,
1
Yuan Wen,
2
,
3
Leigh Ann P. Callahan,
1
,
3
and Gerald S. Supinski
1
,
3
Elizabeth A. Schroder
1Pulmonary Division, Department of Internal Medicine, University of Kentucky, Lexington, Kentucky
2Department of Physiology, College of Medicine, University of Kentucky, Lexington, Kentucky
3Center for Muscle Biology, University of Kentucky, Lexington, Kentucky
Lin Wang
1Pulmonary Division, Department of Internal Medicine, University of Kentucky, Lexington, Kentucky
Yuan Wen
2Department of Physiology, College of Medicine, University of Kentucky, Lexington, Kentucky
3Center for Muscle Biology, University of Kentucky, Lexington, Kentucky
Leigh Ann P. Callahan
1Pulmonary Division, Department of Internal Medicine, University of Kentucky, Lexington, Kentucky
3Center for Muscle Biology, University of Kentucky, Lexington, Kentucky
Gerald S. Supinski
1Pulmonary Division, Department of Internal Medicine, University of Kentucky, Lexington, Kentucky
3Center for Muscle Biology, University of Kentucky, Lexington, Kentucky
 Corresponding author.
Corresponding author.Abstract
Calpain activation has been postulated as a potential contributor to the loss of muscle mass and function associated with both aging and disease, but limitations of previous experimental approaches have failed to completely examine this issue. We hypothesized that mice overexpressing calpastatin (CalpOX), an endogenous inhibitor of calpain, solely in skeletal muscle would show an amelioration of the aging muscle phenotype. We assessed four groups of mice (age in months): 1) young wild type (WT; 5.71 ± 0.43), 2) young CalpOX (5.6 ± 0.5), 3) old WT (25.81 ± 0.56), and 4) old CalpOX (25.91 ± 0.60) for diaphragm and limb muscle (extensor digitorum longus, EDL) force frequency relations. Aging significantly reduced diaphragm and EDL peak force in old WT mice, and decreased the force-time integral during a fatiguing protocol by 48% and 23% in aged WT diaphragm and EDL, respectively. In contrast, we found that CalpOX mice had significantly increased diaphragm and EDL peak force in old mice, similar to that observed in young mice. The impact of aging on the force-time integral during a fatiguing protocol was abolished in the diaphragm and EDL of old CalpOX animals. Surprisingly, we found that CalpOX had a significant impact on longevity, increasing median survival from 20.55 mo in WT mice to 24 mo in CalpOX mice (P = 0.0006).
NEW & NOTEWORTHY This is the first study to investigate the role of calpastatin overexpression on skeletal muscle specific force in aging rodents. Muscle-specific overexpression of calpastatin, the endogenous calpain inhibitor, prevented aging-induced reductions in both EDL and diaphragm specific force and, remarkably, increased life span. These data suggest that diaphragm dysfunction in aging may be a major factor in determining longevity. Targeting the calpain/calpastatin pathway may elucidate novel therapies to combat skeletal muscle weakness in aging.
INTRODUCTION
Aging is known to induce significant skeletal muscle dysfunction, including both the loss of muscle specific force [i.e., force/cross-sectional area (CSA)] and reductions in muscle mass (1–7). Declines in muscle specific force and mass are thought to be major contributors to disability observed with aging. Aging-induced reductions in limb muscle function decrease exercise tolerance and diminish the performance of daily living activities (4, 8–11). Aging also reduces respiratory muscle function (6, 12–16), and, in particular, recent studies indicate that old age is a major risk factor for diaphragm weakness in intensive care unit (ICU) patients (17–23). Diaphragm weakness in critically ill patients, in turn, is now known to be a key determinant of ICU outcomes, markedly increasing the risk of death and prolonged mechanical ventilation (24). As a result, identification of the mechanisms and potential treatments for aging-related limb and diaphragm dysfunction is of enormous clinical importance.
Calpain is a proteolytic enzyme that can cleave multiple myofibrillar components whose activity may be at least partially responsible for aging-induced muscle dysfunction (25–30). Previous works examining the effects of calpain in mediating aging-induced muscle dysfunction have almost exclusively focused on the role of calpain in reducing muscle mass (31–33). Very little is known about the role of calpain in mediating age-related reductions in muscle specific force generation. In addition, almost all previous studies examining the role of calpain in aging-induced muscle dysfunction have been performed on limb muscle and virtually nothing is known about the role of calpain activation in mediating aging-induced reductions in diaphragm function.
The purpose of the present study was, therefore, to examine the mechanism of aging-induced limb and diaphragm muscle dysfunction, including assessment of the role of calpain in mediating reductions in muscle specific force (i.e., force/CSA) and loss of muscle mass. We used muscle-specific inhibition of calpain activity in young and old mice in these studies, namely, utilization of transgenic mice with muscle-specific overexpression of calpastatin (CalpOX), a natural, endogenous calpain inhibitor. We compared groups of wild type (WT) young and old mice with CalpOX young and old mice, measuring limb (extensor digitorum longus, EDL) and diaphragm muscle specific force, repetitive contraction trials, muscle mass, fiber type distribution, muscle fiber type-specific cross-sectional area in the four animal groups. We also monitored activity to determine the role it might play in the measured functional outcomes.
Since skeletal muscle weakness is known to contribute to death in patients with cancer and ICU patients, we also examined life span in our animal colonies of wild type and CalpOX mice. We compared Kaplan–Meier survival curves for WT and CalpOX mice, determining both the median age at the time of death and the maximum age achieved in the two colonies.
MATERIALS AND METHODS
Ethical Approval
All experimental procedures were done in accordance with the institutional guidelines for the care and use of laboratory animals and approved by the University of Kentucky Institutional Animal Care and Use Committee.
Animal Care and Use
Mice with muscle-specific overexpression of calpastatin were shared by Dr. Melissa Spencer and have been previously used in our research studies (34, 35). Briefly, the full-length human calpastatin gene is driven by the human skeletal actin promoter creating a mouse with calpastatin expression solely in skeletal muscle. Mice used in these studies are part of a breeding colony at the University of Kentucky. Mice were housed four to five per cage in individually ventilated caging (IVC). Mice of different sexes were housed separately. Mice had ad libitum access to food and water and lighting was on a 14-h light:10-h dark schedule with the exception of the wheel cage assessment that was performed in a light tight box with a 12-h light:12-h dark lighting schedule. Mice were fed a standard chow diet (2018-Teklad Global 18% Protein Rodent Diet), and cages were changed on the same day each week. To minimize the impact of the housing environment on the aging process, cage changes were the only disturbance to mice in the animal colony as they aged. Male and female mice 4–6 mo of age (young) and 25–27 mo of age (old) were used in these studies (Table 1). All comparisons were made with age-matched, wild type (WT) littermates. Death events were recorded when mice died unexpectedly or were euthanized due to morbidity.
Table 1.
Specifications for mouse groups
| WT Young | CalpOX Young | WT Old | CalpOX Old | |
|---|---|---|---|---|
| Age, mo | 5.87 ± 0.43 (23) | 5.82 ± 0.49 (22) | 25.27 ± 0.58 (22) | 24.91 ± 0.64 (22) |
| Body mass, g | 26.59 ± 0.58 (23) | 27.41 ± 0.61 (22) | 34.82 ± 1.74# (22) | 36.39 ± 1.71# (22) |
| Diaphragm, mg | 37.77 ± 2.41 (10) | 40.62 ± 2.46 (9) | 43.05 ± 1.8 (14) | 45.15 ± 1.91 (13) |
| EDL, mg | 8.22 ± 0.74 (9) | 8.52 ± 0.62 (9) | 7.26 ± 0.37 (8) | 9.54 ± 0.77* (6) |
Data are shown as means ± SE. Numbers in parentheses represent the number of mice used for the individual measurement. CalpOX, overexpression of calpastatin; EDL, extensor digitorum longus; WT, wild type. P < 0.05 was considered statistically significant.
#P < 0.05, strain-matched, young vs. old;
*P < 0.05, age-matched, WT vs. CalpOX.
Western Blot for Calpastatin Levels
We performed Western blots to evaluate levels of calpastatin in the diaphragms of the four study groups. Briefly, diaphragm muscles were homogenized, and protein levels were determined using the Bradford assay (Bio-Rad, Hercules, CA). Western blots were processed using the V3 Western Workflow system and ChemiDoc Touch Imaging System (BioRad, Hercules, CA). Samples were diluted with an equal volume of 2× Laemmli loading buffer, and equal amounts of protein were loaded onto 12% Mini-PROTEAN TGX Stain-Free gels (Bio-Rad). Calpastatin levels were probed with the anti-calpastatin (sc-20779; Santa Cruz Biotechnology, Santa Cruz, CA) antibody. Calpastatin levels were normalized to the total protein loaded for each lane using the Image Lab 5.2.1 software (Bio-Rad). Western blot studies used the tissue collected and frozen at −80°C from mice used in force assessments.
Activity Monitoring
Four-month-old (young) and 26-mo-old (old) male WT and CalpOX mice were placed in light tight boxes in cages fit with voluntary running wheels for 2 wk before activity assessment. The Actimetrics cages consisted of a Tecniplast model 1144B cage bottom (33.2 × 15 × 13 cm) and wire bar lid with an 11-cm (inner diameter) stainless steel wheel, mounted in the cage. Each rotation of the wheel caused a mechanical strike of a switch mounted on the outside of the cage (36). The switch was wired to a computer, and data were recorded with ClockLab software (Actimetrics) (36). Following the acclimation period, activity was measured for 2 wk. Male mice were selected for running wheel assessment for consistency of the data, as female mice tend to run more than male mice and show a greater week to week variability (37). We did not use tissue harvested from these mice for any assessments in this paper, as exercised mice would not be expected to have a similar muscle phenotype to mice without running wheels (sedentary). Even the short duration of exercise used in our study could impact weight, function, and fiber type (n = 9 young WT and CalpOX mice; n = 6 old WT and CalpOX mice).
Force Frequency Curves and Muscle Mass
Muscle specific force generation in the diaphragm and EDL of male and female mice was assessed, as previously reported (35, 38). Left midcostal diaphragm strips or extensor digitorum longus muscle were dissected and mounted vertically in water-jacketed glass organ baths filled with Krebs–Henselheit solution (25°C; NaCl 135 mM, KCl 5 mM, dextrose 11.1 mM, CaCl2 2.5 mM, MgSO4 1 mM, NaHCO3 14.9 mM, NaHPO4 1 mM, and insulin 50 units/L; 95% O2/5% CO2, pH 7.40). One end of each muscle (diaphragm strips or EDL) was attached to the base of an organ bath and the other to a force transducer (Scientific Instruments, Heidelberg, Germany). A biphasic constant current amplifier driven by a Grass S48 stimulator (Grass, RI) delivered supramaximal currents by platinum mesh electrodes. Signals from the Grass stimulator were used to shape the pattern of electrical output delivered to the muscle organ baths by a constant current custom-designed electrical stimulator (Applied Neural Control Laboratory, Case Western Reserve University, with the capacity to deliver up to 10 Amps/channel). To achieve supramaximal stimulation, current levels were adjusted between 600 and 800 mAmp/bath (diaphragm: n = 5 young WT and CalpOX; n = 8 old WT and CalpOX; EDL: n = 5 young WT; n = 4 young CalpOX; n = 4 old WT and CalpOX).
Muscle strips were equilibrated for 15 min before muscle length adjustment to Lo. Muscle strips were stimulated with trains of 1, 10, 20, 50, 100, 150, and 200 Hz (train duration 800 ms, 30 s between adjacent trains). The force-time integral for a repetitive contraction trial (2 min, 40 Hz stimulation, 330 ms duration, 1 train/s) was calculated to evaluate diaphragm and EDL fatigability. The force time integral is an index of the capacity of a muscle to maintain force generation over time during repetitive contractions. A Kipp–Zonen chart recorder was used to record force (NY). Cross-sectional area of the muscle strips was calculated as weight divided by density (1.06 mg mm−3) and length (39), and specific force was calculated as the raw force divided by the cross-sectional area (2, 8). EDL force was corrected for pennation angle (2, 8). EDL was collected and frozen for either biochemical measurements or prepared for histological assessment from the diaphragm studies, and diaphragm was collected and frozen from EDL assessments.
Immunohistochemistry
Diaphragm strips or EDL were carefully dissected and pinned at resting length in a freezing tray, covered with a thin layer of O.C.T. Compound (Tissue‐Tek 4583, Sakura Finetek USA, Inc., Torrance, CA) and snap-frozen in liquid nitrogen. The frozen diaphragm/EDL was stored at −20°C before sectioning or stored long term at −80°C. Muscle samples were sectioned (10-μm thick) and air-dried (40–46), and fiber type immunohistochemistry was performed, as previously described (47). Fiber type was determined by myosin heavy chain isoform expression based on immunoreactivity to specific myosin heavy chain isoform antibodies. Briefly, sections were incubated in antibodies to Type I myosin (BA-D5-c), Type IIa myosin (SC-71-s), laminin (L9393, Sigma), or the absence of immunoreactivity, Type IIb and/or Type IIx. Fluorescent-conjugated secondary antibodies (Invitrogen) against the various mouse immunoglobulin subtypes were applied to visualize myosin and laminin expression. They are as follows: Type I fibers: Goat anti-Mouse IgG2b, Alexa Fluor 647 conjugated 2° Ab (Invitrogen, Cat. No. A21242); Type IIa fibers: Goat anti-Mouse IgG1, Alexa Fluor 488 conjugated 2° Ab (Invitrogen, Cat. No. A21121); and Laminin: Goat anti-Rabbit IgG, AMCA conjugated 2° Ab (1:150) (Vector Lab, Cat. No. Cl-1000). Images were captured with a Zeiss upright microscope (Axio Imager.M1, Zeiss, Göttingen, Germany). Cross-sectional area (CSA) and fiber type distribution were determined by using MyoVision, a software developed and made freely available by the Center for Muscle Biology at the University of Kentucky for automated high-content analysis of skeletal muscle immunohistochemistry (48). The software used laminin immunofluorescence to delineate myofiber membrane and cytoplasmic regions, taking steps to separate connected myofiber regions and filter out longitudinal myofibers that may skew the fiber CSA measurements. The proportion of fiber CSA covered by high immunofluorescence intensity for each fiber type is quantified and used to classify each myofiber as either positive or negative for that fiber type. Manual inspection of the analysis results was performed to exclude immunohistochemical artifacts and tissue damage. Histological data represent n = 5 mice per strain/age for diaphragm and EDL.
Statistical Analysis
Results are reported as means ± 1 SE. One-way and two-way ANOVA were used to determine a significant interaction between factors. Specific statistical tests performed are noted in figure legends. If a significant interaction was detected, a Bonferroni post hoc comparison was performed. P < 0.05 was considered significant. Statistical analysis was performed with Graphpad Prism software.
RESULTS
Body and Muscle Mass
Body mass was measured in the young and old WT and CalpOX mice (Table 1). As expected, aging resulted in a significant increase in body mass in both strains, although no differences were observed between the WT and CalpOX mice at either age. Diaphragm and EDL mass trended higher in the CalpOX mice at both ages; however, only EDL mass in the aged CalpOX mice (7.26 ± 0.37 old WT; 9.54 ± 0.77 old CalpOX) was significantly increased when compared with age-matched WT mice.
Western Blot for Calpastatin Levels
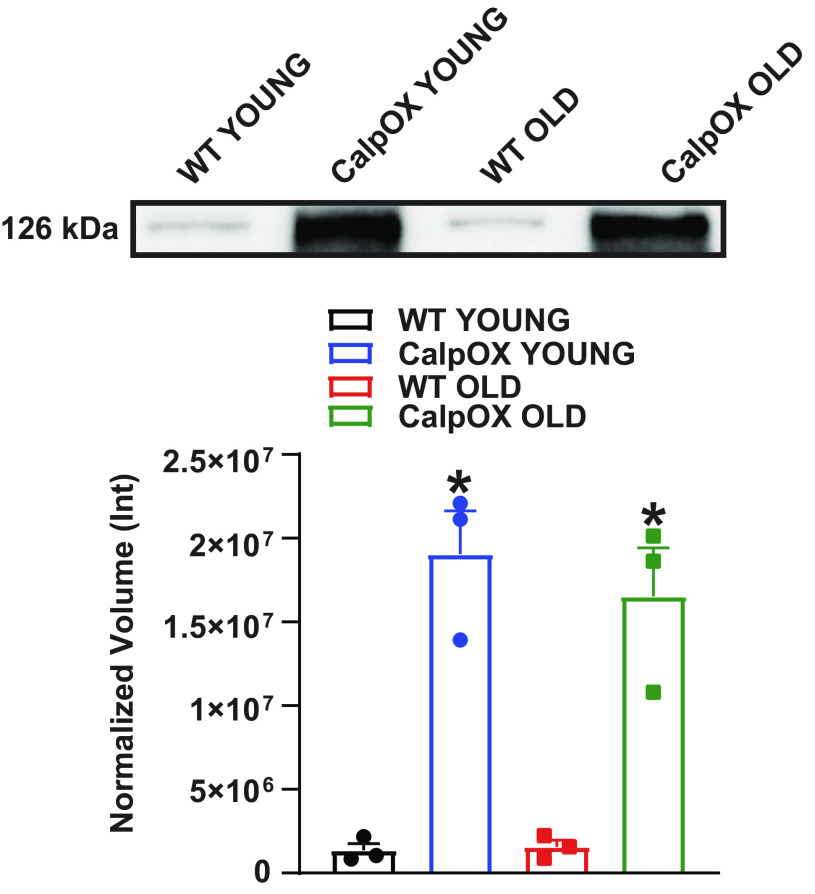
Western blot data showing muscle-specific increases in calpastatin protein expression in young and old CalpOX mice compared with WT mice are shown in Fig. 1 and have been previously demonstrated in our laboratory (35). Western blot data represent n = 3 mice per strain/age.
Western blot data demonstrating overexpression of calpastatin in the diaphragm muscles of CalpOX mice compared with WT mice (n = 3/genotype/age, male). The top panel shows a representative blot, and mean data are presented in the bottom panel. Data are shown as means ± SE. A one-way ANOVA with post hoc Bonferroni analysis was performed. P < 0.05 was considered statistically significant. *P < 0.05, age-matched, WT vs. CalpOX. CalpOX, overexpression of calpastatin; WT, wild type.
Diaphragm Isometric Specific Force
Old age is a major risk factor for diaphragm weakness in intensive care unit (ICU) patients, markedly increasing the risk of death and prolonged mechanical ventilation. To examine the role calpain activation plays in mediating changes in diaphragm function with aging, we measured diaphragm muscle specific force (force/CSA) and force changes in response to a repetitive contraction trial in young and old WT and CalpOX animals. Aging reduced diaphragm specific force compared with young animals (28.56 ± 1.9 N/cm2 young WT; 14.6 ± 1.3 N/cm2 old WT). Importantly, CalpOX had a striking effect to preserve diaphragm specific force with aging (29.29 ± 2.3 N/cm2 young CalpOX; 25.11 ± 1.7 N/cm2 old CalpOX) (Fig. 2, A and andBB).
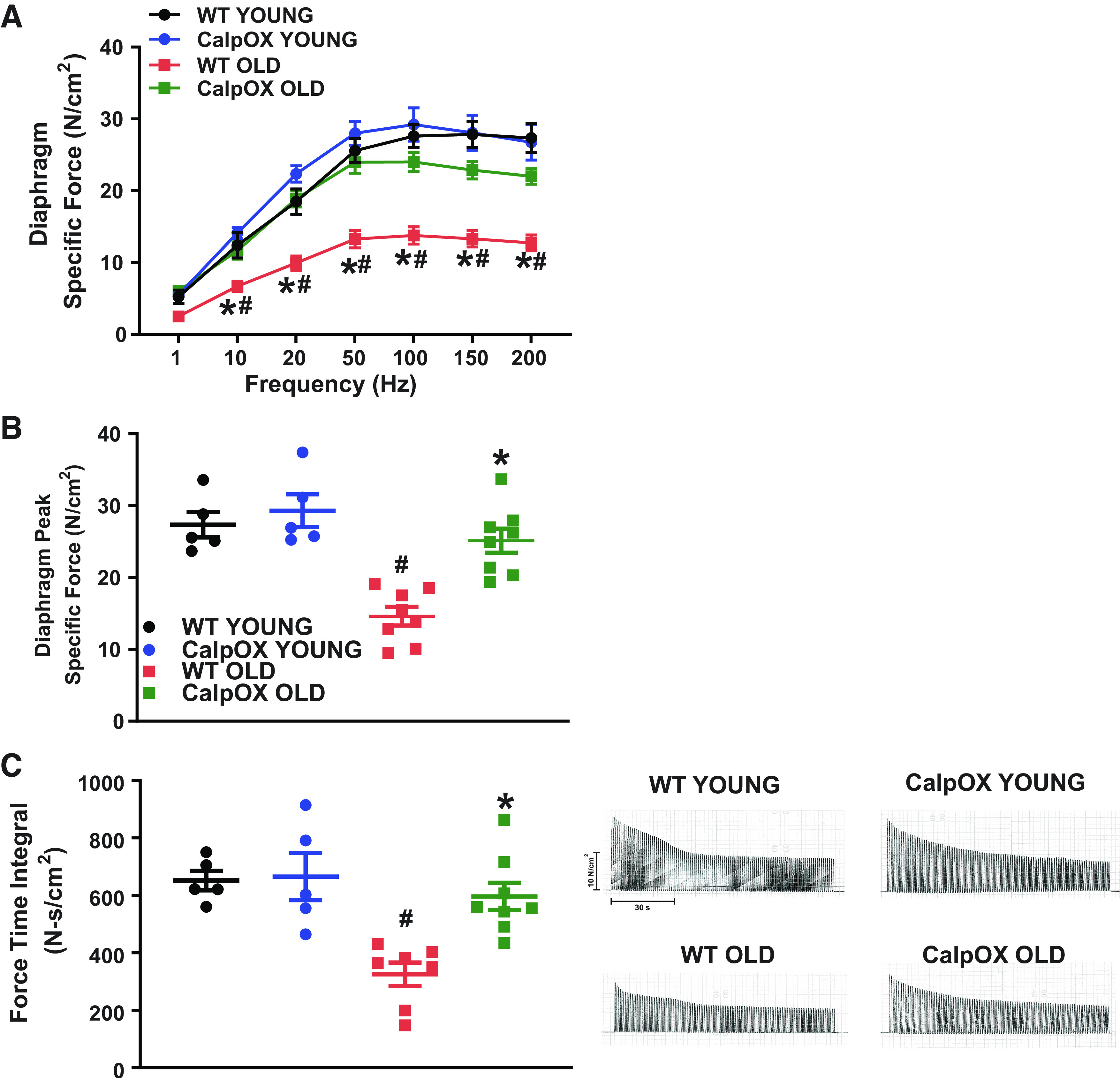
Aging markedly reduced diaphragm specific force as demonstrated by the force frequency relationship which indicates that the specific force in the diaphragm of old WT mice was reduced at all frequencies >10 Hz (A); peak specific force was reduced in the diaphragm of WT mice (B); and the force-time integral for a repetitive contraction trial to evaluate diaphragm fatigability which was significantly reduced in WT mice. These effects were abolished in CalpOX mice. Representative traces are present on the right (C) (n = 4 male and n = 1 female young WT and CalpOX; n = 6 male and n = 2 female old WT and CalpOX). Data are shown as means ± SE. A two-way ANOVA with post hoc Bonferroni analysis was performed. P < 0.05 was considered statistically significant. #P < 0.05, strain-matched, young vs. old; *P < 0.05, age-matched, WT vs. CalpOX. CalpOX, overexpression of calpastatin; WT, wild type.
Aging significantly reduced the force-time integral during a fatiguing protocol by 48%, an effect that was abolished in old CalpOX animals (Fig. 2C). This parameter is thought to be especially important for the diaphragm, which must produce effective repetitive force generation to sustain life.
Diaphragm Fiber Type and CSA
It is well documented that aging alters skeletal muscle fiber size and type (6, 14, 15, 49, 50). We, therefore, determined whether calpastatin overexpression altered the effects of aging on diaphragm cross-sectional area. Using immunohistochemistry, CSA for Type IIb and/or Type IIx fibers in the young CalpOX mice were increased when compared with young WT mice (Fig. 3, A and B). No differences were observed in fiber type distribution when comparing age-matched WT and CalpOX mice (Fig. 3C). The relative contribution of each fiber type to the total diaphragm muscle fiber CSA was calculated as described previously (14, 49). No strain differences were detected; however, there was a decrease in the relative contribution (%) of Type IIb and/or Type IIx fibers in the diaphragm from old mice (both WT and CalpOX) compared with their younger counterparts (Fig. 3D).

Similar trends were present in the CSA and percent fiber type of the diaphragm in both WT and CalpOX mice with age. Representative images (scale = 50 µM) of diaphragm fiber type distribution [immunohistochemistry for myosin isotypes, Type I (pink), Type IIa (green), Type IIb and/or Type IIx (black)] (A), average cross-sectional area of all fibers (B), fiber type distribution (C) in young WT, young CalpOX, old WT, and old CalpOX animals (n = 5 male mice/group), and the relative contribution of fiber types to total diaphragm muscle fiber CSA (D). Data are shown as means ± SE. A two-way ANOVA with post hoc Bonferroni analysis was performed. P < 0.05 was considered statistically significant. #P < 0.05, strain-matched, young vs. old; *P < 0.05, age-matched, WT vs. CalpOX. CalpOX, overexpression of calpastatin; CSA, cross-sectional area; WT, wild type.
EDL Isometric Specific Force
Daily lifestyle and exercise tolerance diminish with aging (10, 11, 37, 51). Therefore, we examined the impacts on limb muscle function. We looked at EDL specific force and found that it was markedly reduced in aging (26.19 ± 1.4 N/cm2 young WT; 15.48 ± 1.4 N/cm2 old WT). Similar to impacts observed in diaphragm, skeletal muscle-specific CalpOX markedly improved aging EDL function (29.6 ± 1.4 N/cm2 young CalpOX; 27.4 ± 1.8 N/cm2 CalpOX old) (Fig. 4, A and B). Analogous to that observed in the diaphragm, aging significantly reduced the force-time integral during a fatiguing protocol by 23%, an effect that was eliminated in old CalpOX animals (Fig. 4C).
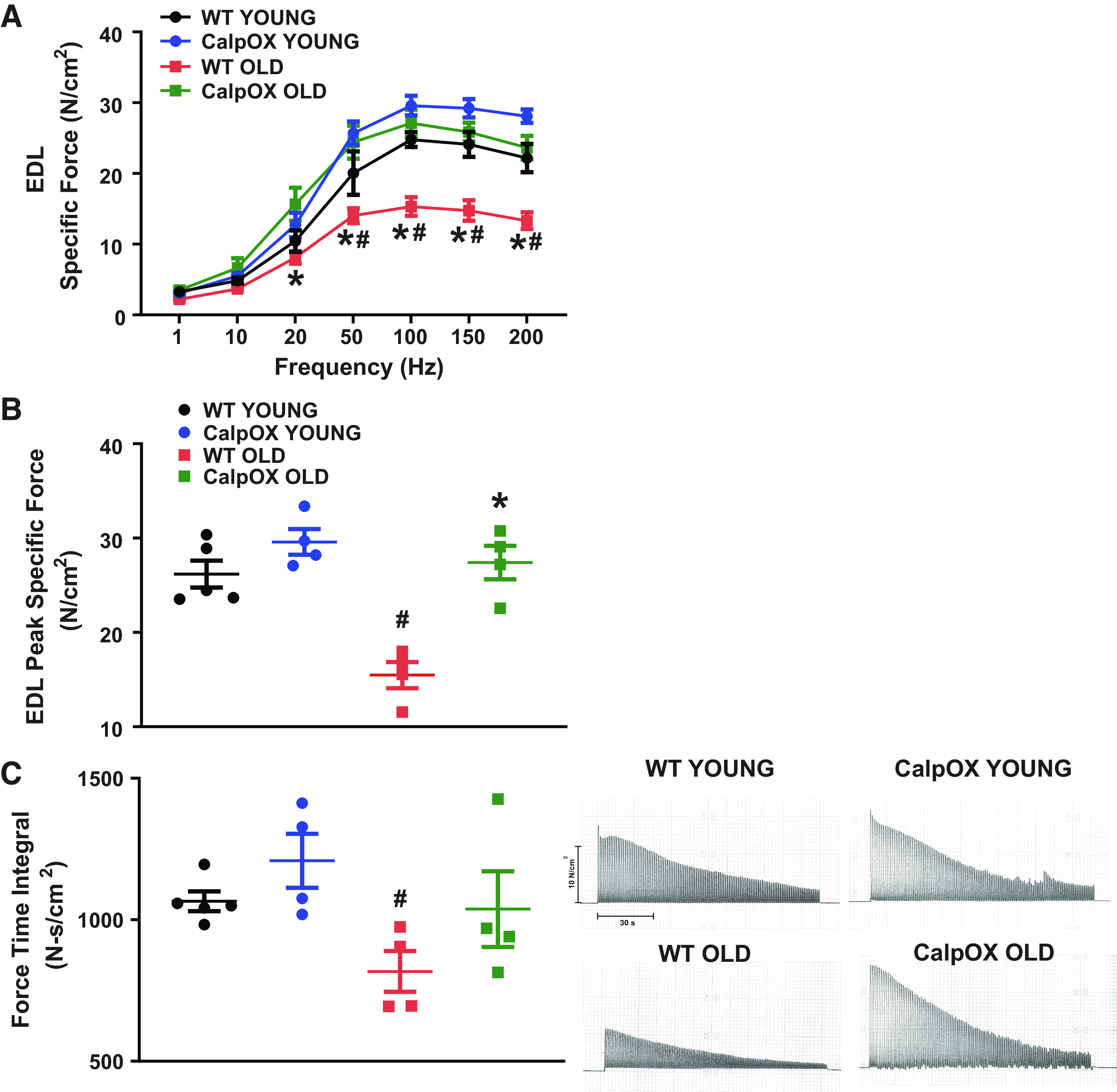
Aging markedly reduced EDL specific force as demonstrated by the force frequency relationship, which indicates that the specific force in the EDL of old WT mice was reduced at all frequencies >20 Hz (A); peak specific force was reduced in the EDL of WT mice (B); and the force-time integral for a repetitive contraction trial to evaluate EDL fatigability which was significantly reduced in WT mice. These effects were abolished in CalpOX mice. Representative traces are present on the right (C) (n = 4 male and n = 1 female WT; n = 4 male young CalpOX; n = 4 male old WT and CalpOX). Data are shown as means ± SE. A two-way ANOVA with post hoc Bonferroni analysis was performed. P < 0.05 was considered statistically significant. #P < 0.05, strain-matched, young vs. old; *P < 0.05, age-matched, WT vs. CalpOX. CalpOX, overexpression of calpastatin; EDL, extensor digitorum longus; WT, wild type.
EDL Fiber Type and CSA
Immunohistochemistry was used to examine CSA and fiber type distribution in the EDL (Fig. 5, A and B). No strain differences were detected between the WT and CalpOX mice EDL CSA and no significant aging impacts were detected in WT mice CSA. CalpOX EDL showed a reduction in Type IIb and/or Type IIx fiber CSA with age. WT mice showed a decrease in the percentage of Type IIb and/or Type IIx fiber types when compared with young WT mice (Fig. 5C). Similar to diaphragm, we calculated the relative contribution of each fiber type to the total EDL muscle fiber CSA (Fig. 5D). This showed a reduction and an increase in the relative contribution of Type IIb and/or Type IIx and Type IIa fibers, respectively, in WT mice when compared with younger counterparts. These differences were ameliorated in the old CalpOX mice.
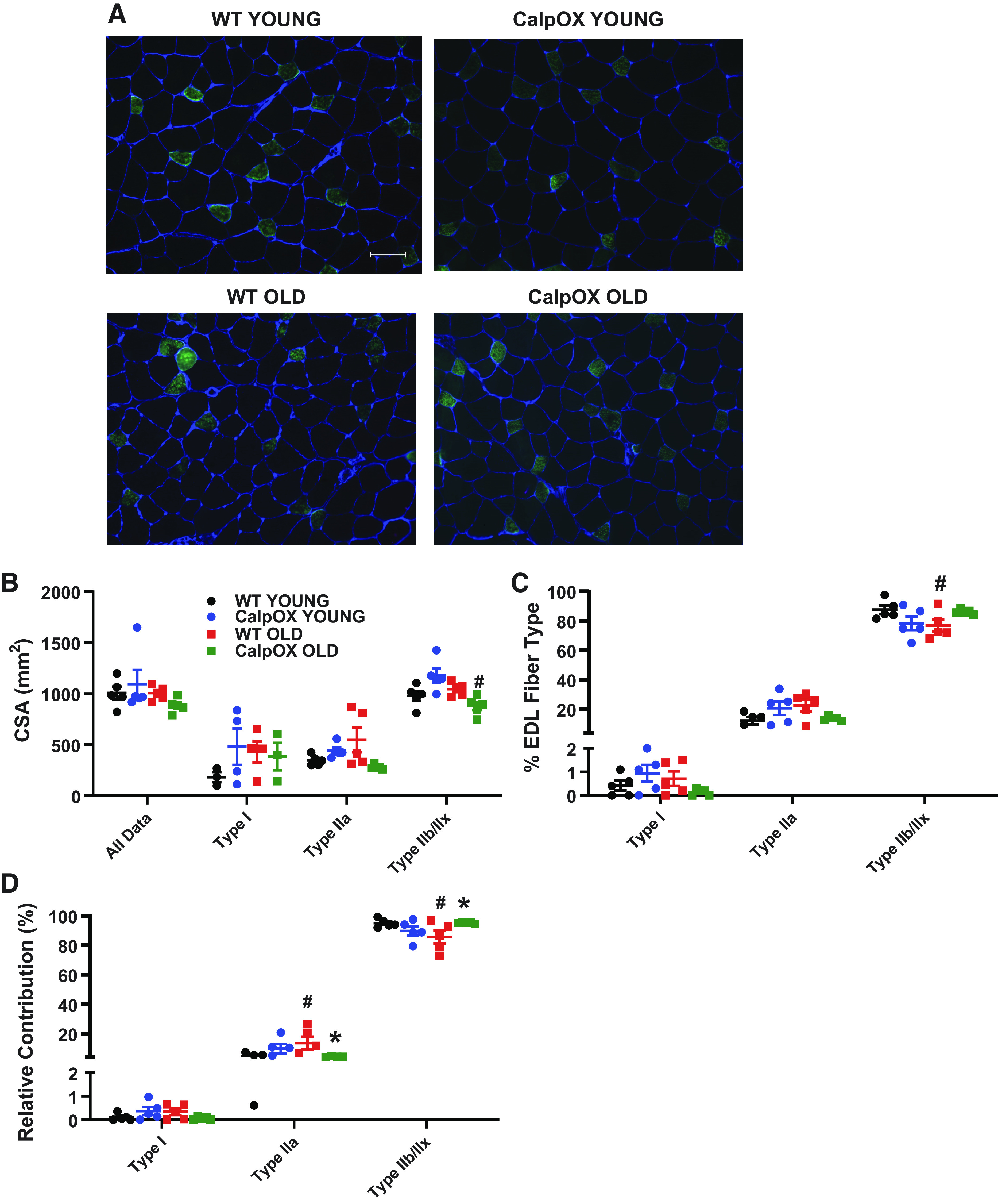
Calpastatin overexpression ameliorated some of the fiber type changes present in the EDL of WT mice. Representative images (scale = 50 µM) of EDL fiber type distribution [immunohistochemistry for myosin isotypes, Type I (pink), Type IIa (green), Type IIb and/or Type IIx (black)] (A), average cross-sectional area of all fibers (B), fiber type distribution (C), and the relative contribution of fiber types to total diaphragm muscle fiber CSA (D) in young WT, young CalpOX, old WT, and old CalpOX animals (n = 5 male mice/group). Data are shown as means ± SE. A two-way ANOVA with post hoc Bonferroni analysis was performed. P < 0.05 was considered statistically significant. #P < 0.05, strain-matched, young vs. old; *P < 0.05, age-matched, WT vs. CalpOX. CalpOX, overexpression of calpastatin; CSA, cross-sectional area; EDL, extensor digitorum longus; WT, wild type.
Activity Assessment
We examined activity in young and old CalpOX and WT mice to determine whether changes in activity might explain the observed differences in muscle function. However, improvements in old CalpOX muscle function could not be explained by effects of calpastatin overexpression to improve activity, as actigraphy data were not different between old WT and old CalpOX animals (Fig. 6, A and B). Average distance, run time, and speed declined consistently in both genotypes with age. These results suggest that inhibition of calpain activation in skeletal muscle improves age-related impairments in limb muscle function.
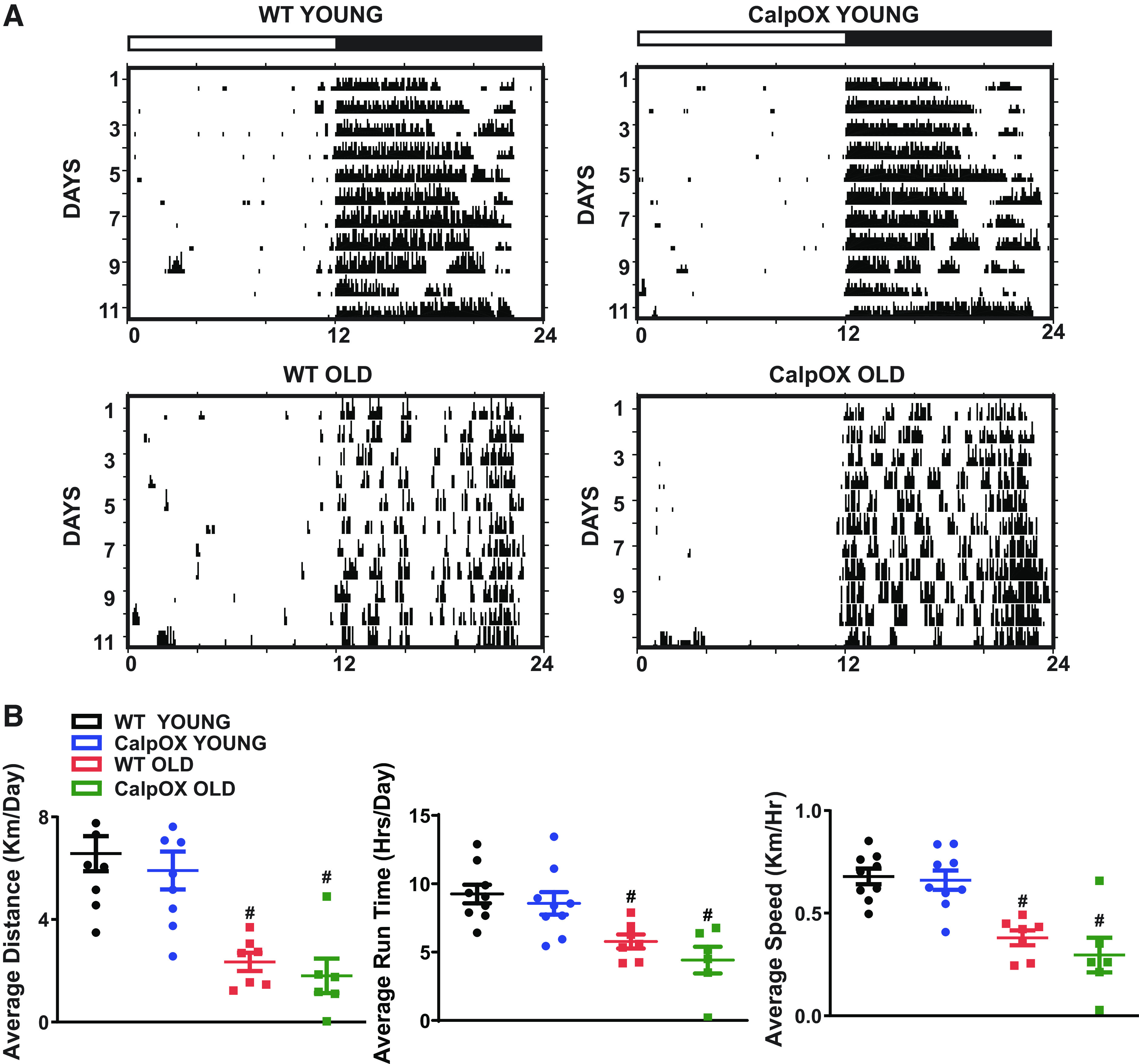
Assessment of activity as shown by representative actograms (A) and average distance run, average run time, and average speed were only different with age (B) (n = 9 young WT and CalpOX; n = 6 old WT and CalpOX). All mice in running wheel experiments were male. Data are shown as means ± SE. A two-way ANOVA with post hoc Bonferroni analysis was performed. P < 0.05 was considered statistically significant. #P < 0.05, strain-matched, young vs. old. CalpOX, overexpression of calpastatin; WT, wild type.
Longevity
We assessed survival in WT and CalpOX animals. The percent survival was 100% for each strain in young mice. The percent survival for each strain in old mice was 23.68% for WT mice and 48.72% for CalpOX mice. Surprisingly, we found that overexpression of calpastatin only in skeletal muscle markedly improved longevity (Fig. 7). Median survival increased from 20.55 mo in WT mice to 24 mo in CalpOX mice (survival curve data: n = 36 WT mice, n = 37 CalpOX mice).
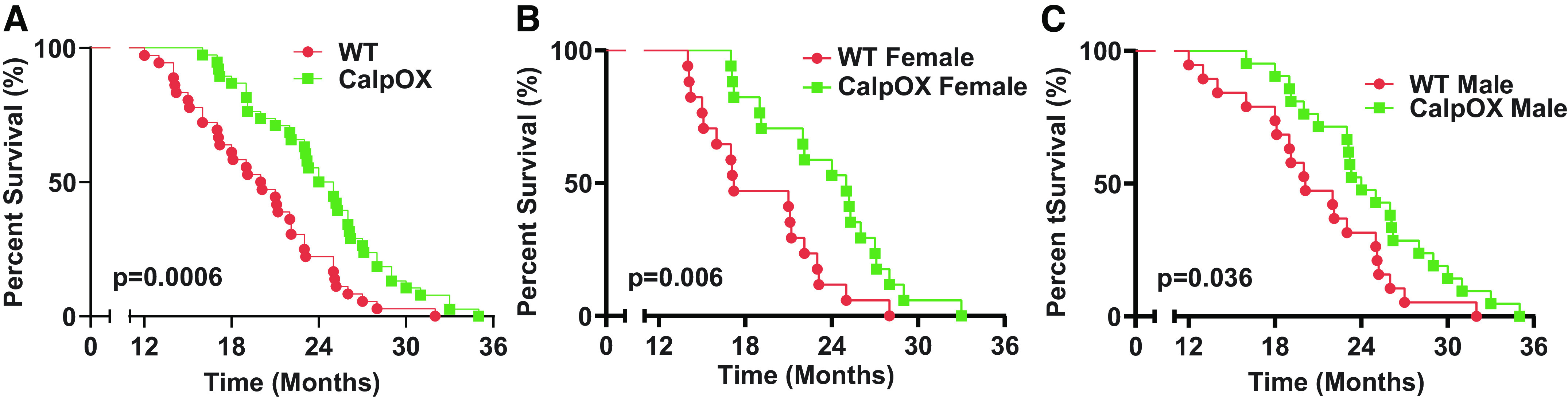
Kaplan–Meier survival curves for WT (n = 38) and CalpOX animals (n = 39). As shown, skeletal muscle-specific overexpression of calpastatin, the endogenous inhibitor of muscle calpain activation, remarkably increased life span (A). This difference was present in both male (C; WT, n = 21; CalpOX, n = 22) and female mice (B; WT, n = 17; CalpOX, n = 17). CalpOX, overexpression of calpastatin; WT, wild type.
DISCUSSION
Aging reduces skeletal muscle function impacting both muscle mass and specific force (6, 8, 9, 13, 14). This natural process can result in decrements of strength with impacts on gait and balance, increasing the risk of falls, as well as interfering with the ability to perform normal daily tasks (1, 5, 52, 53). Numerous studies have demonstrated that limb skeletal muscle size and strength declines with age (54–56). The effect of aging to produce diaphragm weakness (6, 12, 14–16) is of special concern because diaphragm function is an important determinant of outcomes in critically ill patients (21, 22, 24, 57, 58). Specifically, patients that develop life-threatening illnesses almost invariably require mechanical ventilation. Once this form of life support is required, patients with weaker diaphragms require a longer time to achieve liberation from mechanical ventilation and have a much higher incidence of death (24).
Calpains are proteolytic enzymes capable of cleaving multiple cellular proteins, including ATP synthase α, α actinin 3, and myosin heavy chain (31). Importantly, several previous studies suggest a potential role for calpain activation as a contributor to aging-induced muscle dysfunction. For example, Fraysse et al. (59) found that resting intracellular muscle calcium levels, a central modulator of calpain activation, are increased in aged muscle. In another study, Dargelos et al. (60) measured calpain properties in muscles of young and old animals and found increases in calpain enzyme activity levels with aging. A single previous study used transgenic mice with muscle-specific overexpression of calpastatin, the natural endogenous inhibitor of calpain, and found that aging-induced reductions in skeletal muscle mass were attenuated in muscle-specific calpastatin overexpressing mice compared with wild type mice (32).
None of these studies, however, examined the role of calpain activation in modulating aging-induced reductions in muscle specific force generation (i.e., muscle force/cross-sectional area). The current work, to our knowledge, is the first study to examine the effects of aging on muscle specific force using transgenic mice with muscle-specific overexpression of calpastatin. This mouse model is especially useful for studies examining muscle calpain activation, because it inhibits calpain with molecular specificity, whereas all chemical calpain inhibitors have, to greater or lesser degrees, effects on other proteolytic enzyme systems. This mouse model also provides tissue specificity, as calpain is inhibited only in skeletal muscle, whereas systemic administration of chemical calpain inhibitors affects all organs, with the potential for off-target effects on nerves, blood flow, and hormones that may nonspecifically influence muscle parameters.
Importantly, we found that aging induced severe reductions in both maximal specific force (i.e., in response to high stimulation frequencies, 50–150 Hz.) and submaximal specific force (i.e., stimulation frequencies between 1 and 20 Hz) in both the diaphragm and extensor digitorum muscles (EDL). In addition, we found that muscle-specific calpastatin overexpression prevented the effects of aging on specific force, preserving both maximal and submaximal levels of specific force in both the diaphragm and EDL muscles. These findings are consistent with a major role for calpain in modulating aging-induced decrements of muscle specific force in both the diaphragm and EDL muscles.
Only one previous study found that a chemical calpain inhibitor, namely, BDA-410, modestly improved soleus low-frequency specific force (1–20 Hz force increased by 20%) but had no significant effect on maximal specific force (i.e., in response to high stimulation frequencies), muscle mass, fiber composition, or myosin heavy chain levels (33). Of note, the effect of aging to increase muscle calcium levels varies from muscle to muscle, with little increase in the soleus (59), potentially accounting for the small responses observed by Zhang et al. (33). Muscle-specific calpastatin overexpression allows for molecular and tissue specific inhibition of calpain avoiding the nonspecific actions of chemical calpain inhibitors like BDA-410.
Diaphragm fiber type and CSA were examined to determine whether calpastatin overexpression might play a role in mediating the effects of aging on muscle mass or muscle specific force generation (8, 12, 14–16, 50, 61–63). Although no significant differences in CSA or % fiber type were observed in WT diaphragm with aging, we did observe a decrease in the relative contribution of Type IIb and/or Type IIx fibers, which is consistent with previous studies in the diaphragm (14, 64). Aside from an increase in Type IIb and/or Type IIx fiber CSA when comparing young WT and CalpOX mice, overall trends were similar between the two strains, suggesting that the main role of calpain in mediating aging-induced muscle dysfunction is to reduce muscle specific force generation and not to reduce muscle mass.
In the EDL, calpastatin overexpression appeared to modulate aging-induced alterations in muscle. The percentage of Type IIb and/or Type IIx fibers decreased in the old WT mice. A decrease was also observed for Type IIb and/or Type IIx fiber type CSA in CalpOX mice with age. We calculated the relative contribution of fiber types to overall EDL CSA. This data revealed typical age-related increases and decreases in the relative contribution of Type IIa and Type IIb and/or Type IIx fibers, respectively, in WT mice that were ameliorated in CalpOX EDL. These differences may contribute to the preservation of force in aging CalpOX EDL.
The most striking finding in this study, however, is related to our data demonstrating that skeletal muscle-specific calpastatin overexpression markedly increased survival. Across life span, 9 mouse days are equivalent to 1 human yr (65) and postsenescence, 2.07 days are equivalent to 1 yr; this increased longevity would equate to an astonishing increase in survival of ∼10–35 yr in humans (66). To our knowledge, only a few limited studies have demonstrated that skeletal muscle-specific alteration of a single gene/protein significantly increases life span in aged mice and none has impacted life span as markedly (67–69). These findings raise the question as to how improving aging-induced skeletal muscle dysfunction could have such a dramatic effect on longevity. It is difficult to explain increased survival based solely on improvements in limb muscle function. On the other hand, diaphragm weakness, a major cause of death in a number of conditions including sepsis (22, 24, 35, 70, 71), cancer (72, 73), and many neuromuscular diseases (74, 75), is markedly improved with CalpOX. We, therefore, speculate that the major effect of skeletal muscle-specific overexpression of calpastatin on survival is due to restoration of diaphragm function. If so, diaphragm dysfunction in aging may be a major factor in determining longevity.
There seem to be three possible explanations with precedent in other mouse models or human studies for the association between diaphragm function and longevity: 1) the effect of calpastatin overexpression to increase diaphragm function may delay or prevent death from certain diseases (e.g., sepsis, cancer) (21, 24, 72); 2) muscle calpastatin expression may alter release of muscle mediators (e.g., exosomes, myokines) that have systemic effects on other organs, increasing survival (76–80); and 3) calpastatin-dependent increases in muscle function may promote increases in activity across life span which, in turn, improves survival (11, 77, 81–83). The experiments presented here represent the first step in elucidating the relative merits of these competing possibilities, with an emphasis on examining the link between calpastatin-dependent improvements in diaphragm function and survival.
There are a few limitations to the described studies. First, mice are not humans, and there is no perfect way of replicating the life cycle of humans in animal models, especially in a rodent model that lives on average ∼2 yr. In addition, our mice were from an in-house breeding colony and housed within our animal facility. To mitigate environmental impacts, we attempted to limit disturbance to our colony throughout the aging process, only opening cages once a week during cage changes. Daily visual inspections were performed without cage disturbance. However, we are aware that aging studies should ideally limit pathogen exposure in barrier housing as environmental pathogens have been shown to impact longevity (84–86). Another limitation results from our choice of study timepoint in the aging mice. The percent survival for each strain at ∼26 mo of age (average study age, Table 1) is 23.68% for WT mice and 48.72% for CalpOX mice. Longevity studies of biological aging should avoid extremes in aging (very young and very old). This is particularly important for mice in the last surviving third of a population, as assessments may be impacted by illness present in the aged mice (87). Our aging timepoint was selected based on previous literature showing a ∼50% survival in B6 (background strain) mice at 26 mo of age (86). The mouse used in this study was a transgenic. Although transgenic models are beneficial for interrogating the function of a specific gene, pharmacological therapies for the purpose of reversing aging-induced skeletal muscle weakness by augmenting calpastatin expression in muscle will need to be devised. Calpastatin overexpression may increase diaphragm strength and survival, but these two phenomena may not be causally linked. The fact that recent data support the concept that diaphragm strength may be a major factor in determining death from sepsis (22, 24, 35, 70, 71) and cancer (72, 73, 88) support a causal relationship between diaphragm weakness and death. Our study did not evaluate neuromuscular transmission that may be another site of calpain action. Blockade of calpain activity at the neuromuscular junction by calpastatin improves neuromuscular transmission and strength in myasthenic mice (89). Our findings are consistent with many studies indicating that muscle force production in aging is not solely dependent on muscle size, but most likely the result of age-related changes in the properties of both the skeletal muscle and nerves that innervate them (8, 44, 50, 53, 61, 83). Biological changes that occur with aging in the absence of obvious changes in muscle mass are related to muscle quality. Our in vitro force assessment data support improved muscle quality in the aged CalpOX mice, and by extension, suggest a role for calpastatin in maintaining muscle quality in aging.
In summary, to our knowledge, this is the first study to investigate the role of calpastatin overexpression on skeletal muscle specific force in aging rodents. We found that muscle-specific overexpression of calpastatin, the endogenous calpain inhibitor, prevented aging-induced reductions in both EDL and diaphragm specific force, preserving force in response to both low- and high-frequency electrical stimulation. We also found that muscle-specific calpastatin overexpression increased life span. We speculate that the major effect of skeletal muscle-specific overexpression of calpastatin on survival is due to the restoration of diaphragm function. If true, diaphragm dysfunction in aging may be a major factor in determining longevity. Our data provide a strong rationale that targeting the calpain/calpastatin pathway may elucidate novel therapies to combat skeletal muscle weakness in aging and by extension, enhance longevity.
GRANTS
G. S. Supinski is supported by R01HL113494 and R01HL141356 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) and 5I01BX002132 from the Department of Veterans Affairs. L. A. Callahan is supported by R01HL112085 and R01HL141356 from the National Heart, Lung, and Blood Institute of the NIH. E. A. Schroder is supported by R01HL141356 from the National Heart, Lung, and Blood Institute of the NIH.
AUTHOR CONTRIBUTIONS
E.A.S., L.A.P.C., and G.S.S. conceived and designed research; E.A.S., L.W., and G.S.S. performed experiments; E.A.S., L.W., Y.W., and G.S.S. analyzed data; E.A.S., L.A.P.C., and G.S.S. interpreted results of experiments; E.A.S. prepared figures; E.A.S., L.A.P.C., and G.S.S. drafted manuscript; E.A.S., L.W., Y.W., L.A.P.C., and G.S.S. edited and revised manuscript; E.A.S., L.W., Y.W., L.A.P.C., and G.S.S. approved final version of manuscript.