- Journal List
- Exp Ther Med
- v.16(4); 2018 Oct
- PMC6143843

Aerobic exercise-stimulated Klotho upregulation extends life span by attenuating the excess production of reactive oxygen species in the brain and kidney
Naichun Ji
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Jing Luan
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
2Institute of Holistic Integrated Medicine, Shaanxi University of Chinese Medicine, Xi'an, Shaanxi 712046, P.R. China
Fengrui Hu
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
2Institute of Holistic Integrated Medicine, Shaanxi University of Chinese Medicine, Xi'an, Shaanxi 712046, P.R. China
Yirong Zhao
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Bosen Lv
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Wen Wang
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Meng Xia
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Xin Zhao
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Kejing Lao
1Department of Physical Education and Shaanxi Key Laboratory of Brain Disorders and Institute of Basic and Translational Medicine, Xi'an Medical University, Xi'an, Shaanxi 710021, P.R. China
Associated Data
- Data Availability Statement
All data generated or analyzed during the present study are included in this published article.
Abstract
Aerobic exercise induces many adaptive changes in the whole body and improves metabolic characteristics. Klotho, an anti-aging gene, is mainly expressed in the brain and kidney. The roles of Klotho in the brain and kidney during aerobic exercise remain largely unknown. The present study aimed to determine whether aerobic exercise could influence the expression of Klotho, decrease reactive oxygen species (ROS) and prolong life span. Sprague Dawley rats were exercised on a motor treadmill. Klotho mRNA and protein expression levels in rat brain and kidney tissues were examined using reverse transcription-quantitative polymerase chain reaction and western blotting, respectively. ROS production was detected following intermittent aerobic exercise (IAE) or continuous aerobic exercise (CAE). Kaplan-Meier curve analysis demonstrated that aerobic exercise significantly improved rat survival (P<0.001). The ROS levels in rat brain and kidney tissues were decreased in the aerobic exercise groups compared with the control group (P<0.05). In addition, Klotho mRNA and protein expression levels were increased significantly following aerobic exercise compared with controls (P<0.05). There was no significant difference between the IAE and CAE groups in any experiments (P>0.05). These results suggest that aerobic exercise-stimulated Klotho upregulation extends the life span by attenuating the excess production of ROS in the brain and kidney. As Klotho exhibits a potential anti-aging effect, promoting Klotho expression through aerobic exercise may be a novel approach for the prevention and treatment of aging and aging-related diseases.
Introduction
Aging (physiological and pathological) refers to the gradual decline of various organ functions of the body. Aging is mainly regulated by the body's genetic information. However, external factors, such as bad living habits and environmental factors, are also associated with the aging of the body. In contrast, good habits and healthy practices, such as moderate exercise, can effectively delay aging and improve quality of life (1,2).
Aerobic exercise refers to the physical exercise performed by the human body with a full supply of oxygen. During aerobic exercise, oxygen uptake and oxygen demand in the body are equal (3). As the body obtains energy via the aerobic oxidation of energy substances and that the energy required for exercise must be provided by oxidizing starch fat and protein in the body, aerobic exercise requires a duration of at least 30 min (3,4). In addition, the exercise intensity is in the middle (120–150 beats/min) or upper middle level (>151-180 beats/min), the athlete's heart rate is maintained at 60–80% of the maximum heart rate (4). According to the exercise method, aerobic exercise is divided into continuous aerobic exercise (CAE) and intermittent aerobic exercise (IAE). Aerobic exercise is important for improving body functions and quality of life (3).
Klotho is an anti-aging gene that encodes a type I transmembrane protein. It is highly expressed in the cerebral choroid plexus and renal tubular epithelia (5). As a membrane-bound protein, Klotho can be cleaved; Klotho has also been identified as a soluble protein in the blood, urine and cerebrospinal fluid (6,7). Klotho protein participates in many pathways that govern aging, such as regulation of phosphate homeostasis, insulin signaling and Wnt signaling (8). In mammals, the body gradually undergoes an aging process over time. The expression of Klotho also gradually declines (9). In animal experiments, overexpression of Klotho protein delays aging and prolongs life span (10,11). In addition, it has previously been demonstrated that Klotho protein increases the ability of cells to remove reactive oxygen species (ROS) (12). In cell culture experiments and transgenic mice, it was also demonstrated that Klotho protein enhances the resistance of cells to ROS and prolongs the life span (13). However, it remains to be elucidated whether, under conditions of aerobic exercise, Klotho-mediated signaling pathways also contribute to the enhanced resistance of cells to ROS and to a prolonged life span.
In the present study, two aerobic exercise models were used in Sprague Dawley (SD) rats to investigate whether aerobic exercise influenced body functions and prolonged life span by regulating Klotho-mediated signaling pathways. The aim of the study was to elucidate the association among the Klotho gene, aerobic exercise, and aging.
Materials and methods
Experimental animals and aerobic exercise models
Male 3-month-old (weight, 320–350 g) SD rats were purchased from the Animal Experimental Center at the Medical School of Xi'an Jiaotong University (Xi'an, China) and maintained in microisolator cages in a specific-pathogen-free facility. All rat experiments were performed using protocols approved by the Institutional Ethics Committee on Animal Use of Xi'an Medical University (Xi'an, China). The temperature of the breeding environment was 20–25°C, the relative humidity was 50–70%, and the light/dark cycle was set at 12-h/day. Experimental rats were fed with national standard rodent feed and autoclaved water ad libitum.
The total number of rats used in the present study was 101. After they had adapted to the environment for one week, 30 rats were selected randomly as two control groups (one group included 22 rats for survival analysis and the other one included 8 rats for tissue detection) where these rats were not subjected to any type of exercise regime. The other rats received adaptive training as follows: 10–15 m/min for 30 min/day for 5 days. A total of 8 rats that failed to qualify for the exercise training were removed, and the remaining 63 rats were randomly divided into four groups, including two IAE groups (one group included 22 rats for survival analysis and the other one included 9 rats for tissue detection) and two CAE groups (one group included 22 rats for survival analysis and the other one included 10 rats for tissue detection). The CAE model was established following a previously published protocol (14). The specific steps were as follows: The treadmill slope was 0°; the training speed was 16 m/min [50–60% maximal oxygen uptake (VO2 max)]; and the training time was 60 min/day for 5 days/week for a total of 48 weeks (15,16). The IAE model was established following a previously published protocol (17). The specific steps were as follows: The treadmill slope was 0°; the training program was 10 m/min (40–50% VO2 max) exercise for 10 min, 25 m/min (80–90% VO2 max) exercise for 7 min, and 15 m/min (50–60% VO2 max) exercise for 3 min; and the training time was 60 min/day alternated with the above three protocols for 5 days/week for a total of 48 weeks.
Survival analysis
Following aerobic exercise for 48 weeks, 3 groups of rats, including the control group, an IAE group, and a CAE group, continued to be fed routinely and were observed daily until the end of their lives. The survival time of the rats was then analyzed.
Animal tissues
Following the 48-week aerobic exercise program, rats in the other three groups (control, IAE and CAE) were sacrificed with barbiturate (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) overdose (150 mg/kg) via intraperitoneal injection. Rat kidney and brain tissues were quickly removed and stored at −80°C for later use.
Determination of ROS in renal and brain tissues
ROS levels were measured using a high quality green fluorescence assay kit (GMS10016.3; GenMed Scientifics Inc., Shanghai, China). Quantitative detection of tissue homogenate was performed according to the manufacturer's protocol. Briefly, 50 mg kidney and brain tissues from each rat was collected and homogenized. Subsequently, 5 µl of final tissue homogenate was used for quantitative protein detection. Protein quantification was performed using the GENMED Bradford kit (GMS30030.1; GenMed Scientifics Inc.) according to the manufacturer's protocol. Based on those results, the volume of final homogenate containing 100 µg protein was calculated. The corresponding volume of the final homogenate was then mixed with the detection reagent. The mixture was incubated at 37°C in the dark for 20 min and tested immediately. The relative fluorescence unit (RFU) values were measured at 490/520 nm using a multifunction fluorescence microplate reader, and the result was expressed as the RFU value of the assay well minus the RFU value of the blank well.
mRNA expression and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
A 50 mg quantity of rat kidney and brain tissue was collected, and total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The purity and concentration of the extracted total RNA were determined via an ultraviolet spectrophotometer (UV1800; Shimadzu Corporation, Kyoto, Japan). A PrimeScript™ RT reageant kit (Takara Biomedical Technology Co., Ltd., Beijing, China) and a PCR amplification apparatus (Eppendorf, Hamburg, Germany) were used to reverse transcribe the RNA into cDNA. The RT temperature protocol was 42°C for 15 min, 85°C for 5 sec and standby at 4°C; then, the mRNA expression levels of Klotho were measured using a thermal cycler (ABI Veriti FAST thermal cycler; Thermo Fisher Scientific, Inc.). SYBR Green Real-time PCR Master Mix was purchased from Takara Biomedical Technology Co., Ltd.
The upstream primer sequence of the Klotho gene was 5′-ATCCGGCCTCAGATAACCTT-3′. The downstream primer sequence of the Klotho gene was 5′-CCACCACTGGAGTGATGTTG-3′. The product length was 175 bp. The reference gene was rat GAPDH. The upstream primer sequence of rat GAPDH gene was 5′-GGTGGACCTCATGGCCTACA-3′. The downstream primer sequence of GAPDH gene was 5′-CTCTCTTGCTCTCAGTATCCTTGCT-3′. The product length was 200 bp. The Applied Biosystems® 7500 SDS v2.0.6 Software (Thermo Fisher Scientific, Inc.) that comes with the PCR instrument was used to read and analyze the target gene quantitative CT values. The reaction system was as follows: 2×SYBR Green Real-time PCR Master Mix (Modified DNA polymerase, SYBR Green I, Optimized PCR buffer, 5 mM MgCI2, dNTP mix including dUTP; Toyobo Life Science, Osaka, Japan) 10 µl, DEPC water 8 µl, upstream primer 0.5 µl, downstream primer 0.5 µl, cDNA 1 µl. The thermocycling conditions were as follows: 95°C for 3 min, 95°C for 30 sec and 60°C for 30 sec for 35 cycles, followed by 72°C for 5 min ad holding at 4°C for further use.
Western blot analysis
A 100-mg sample of kidney tissue and brain tissue was taken from each rat, minced, and ground with liquid nitrogen. Then, 1 ml radioimmunoprecipitation assay protein lysis buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was added, and the samples were kept on ice for 10 min. The samples were subsequently centrifuged at 12,000 × g for 5 min at 4°C and the total protein concentration in the clear supernatant was evaluated using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). Aliquots containing 20 µg protein were subjected to 10% SDS-PAGE. The proteins were then transferred to a polyvinylidene difluoride membrane. Membranes were blocked for 1 h at room temperature with a 5% skim milk in Tris buffered saline. The membranes were washed with TBS buffer three times for 5 min per wash. The primary antibodies [murine anti-GAPDH (ab8245; 1:10,000, Abcam, Cambridge, UK) and rabbit-derived anti-Klotho (ab203576; 1:2,000, Abcam)] were blocked in 10 ml of 5% skim milk and incubated at 4°C overnight. The membranes were washed twice for 10 min/wash with Tween-20 TBS buffer. The horseradish peroxidase-labeled secondary antibodies (cat. no. ab6728 for Klotho and ab6721 for GAPDH; 1:10,000, Abcam) were added to 5 ml of 5% skim milk and incubated for 2 h at 37°C. The membranes were washed twice for 10 min/wash with Tween-20 TBS buffer. Specific signals were detected with enhanced chemiluminescence solution (Thermo Fisher Scientific Inc., Waltham, US). Densitometry was performed using Image Lab 4.1 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and the density of each band was normalized against that of GAPDH. All experiments were performed in triplicate.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk, NY, USA) was used for data analysis. All data are expressed as the mean + standard deviation. Log-rank test was used in the survival analysis of the rats in Fig. 1A. One-way analysis of variance was used for data in Figs. 1B and and22–4 followed by a Student-Newman-Keul's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
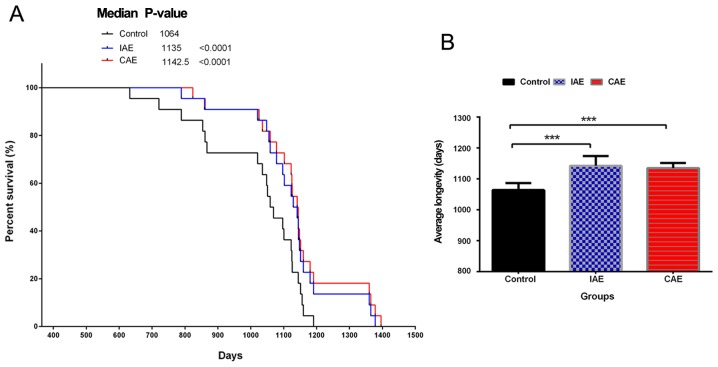
Aerobic exercise prolonged the survival time of rats. Following 48 weeks of aerobic exercise, three groups of rats, including the control, IAE and CAE groups, continued to be fed routinely and were observed daily until the end of their lives. (A) Kaplan-Meier curves of rat survival. (B) The average lifespan of rats in different groups. Data are presented as the mean + standard deviation (n=22/group). ***P<0.001 vs. control group. IAE, intermittent aerobic exercise; CAE, continuous aerobic exercise.
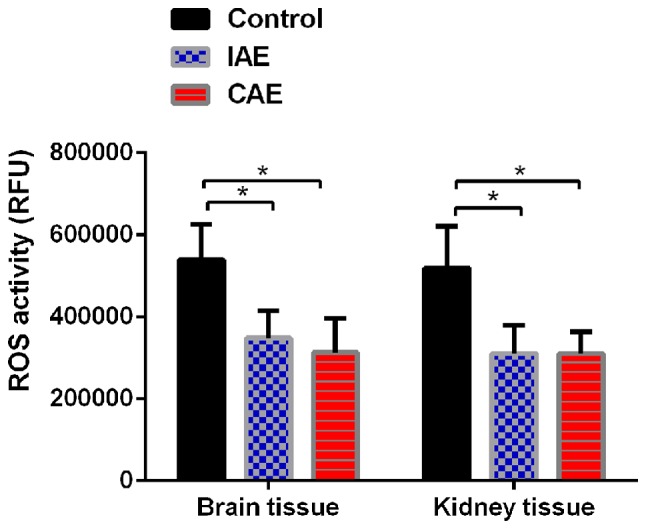
Aerobic exercise decreased ROS levels in rat kidney and brain tissues. ROS levels in rat kidney and brain tissues were tested using an ROS kit. The RFU values were measured at 490/520 nm. Each result was expressed as follows: RFU value of the assay well - the RFU value of the blank well. Data are presented as the mean + standard deviation (n=8 in control group, n=9 in IAE group and n=10 in CAE group). *P<0.05 vs. control group. ROS, reactive oxygen species; IAE, intermittent aerobic exercise; CAE, continuous aerobic exercise.
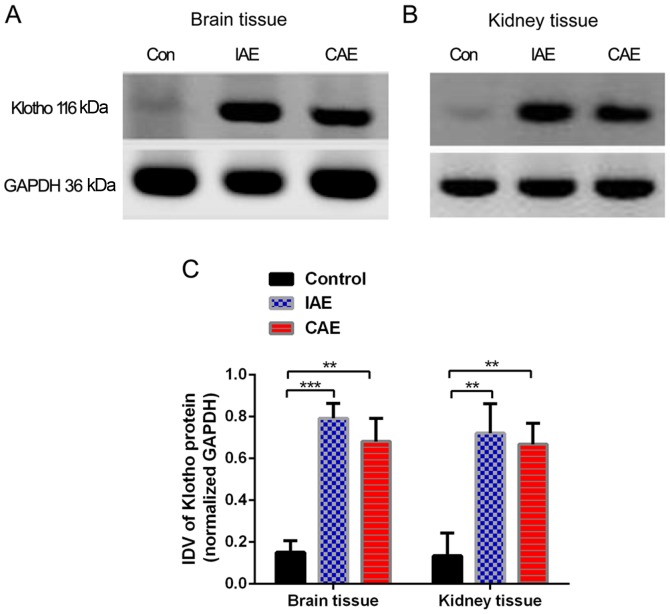
Aerobic exercise increased the Klotho protein levels in rat brain and kidney tissues. Protein levels in rat (A) brain and (B) kidney tissues were evaluated via western blot analysis. (C) IDVs of western blot results for brain and kidney tissues are presented. Protein levels were normalized to those of GAPDH. The relative level of protein in the control group was set at 100%. **P<0.01 and ***P<0.001 vs. control group. Data are presented as the mean + standard deviation of triplicate results (n=8 in control group, n=9 in IAE group and n=10 in CAE group). IDV, integrated density value; IAE, intermittent aerobic exercise; CAE, continuous aerobic exercise; Con, control.
Results
Aerobics exercise prolongs the survival time of rats
To determine whether aerobics exercise could influence physical quality and prolong the survival time, the rats in different groups were observed daily until the end of their lives (Fig. 1). Kaplan-Meier curve analysis demonstrated that in both the IAE and CAE groups, the survival time of rats was significantly prolonged compared with that in the control group (P<0.0001; Fig. 1A). The mean survival time of rats in the IAE and CAE groups was 1,142.5±32.0 and 1,135±17.0 days, respectively. The mean survival time in the control group was only 1,064±23 days. Aerobic exercise increased the mean survival time of rats by >70 days compared with that in the control group (P<0.0001; Fig. 1B). There was no significant difference between the IAE and CAE groups. The above results demonstrated that aerobic exercise could improve physical quality and extend life expectancy.
Aerobic exercise decreases ROS levels in rat kidney and brain tissues
To determine whether aerobic exercise could increase the clearance rate of ROS, the ROS levels in rat kidney and brain tissues were evaluated. As presented in Fig. 2, following aerobic exercise for 48 weeks, ROS levels were significantly decreased in brain and kidney tissues of IAE and CAE rats compared with those of control rats (P<0.05). The ROS levels in the brain tissues of the IAE rats were reduced to 64% of those of the control rats, and the ROS levels in the brain tissues of the CAE rats were reduced to 58% of those of the control rats. Similarly, the ROS levels in the kidney tissues of the IAE rats were decreased to 60% of those of the control rats, and the ROS levels in the kidney tissues of the CAE rats were decreased to 59% of those of the control rats. There was no statistically significant difference between IAE and CAE rats (P>0.05).
Aerobic exercise upregulates the mRNA expression levels of Klotho in rat brain and kidney tissues
Following aerobic exercise for 48 weeks, mRNA levels of Klotho in the rat brain and kidney tissues, as detected by RT-qPCR, were significantly increased in the IAE and CAE groups compared with the control group (P<0.05; Fig. 3). The mRNA levels of Klotho were increased by 1.71-fold in the brain tissues of IAE rats compared with those of control rats, and ROS levels were increased by 1.64-fold in the brain tissues of CAE rats compared with those of control rats. Similarly, ROS levels were decreased by 2.12-fold in the kidney tissues of IAE rats compared with those of control rats, and ROS levels were decreased by 1.97-fold in the kidney tissues of CAE rats compared with those of control rats. There was no statistically significant difference between the IAE and CAE groups (P>0.05).
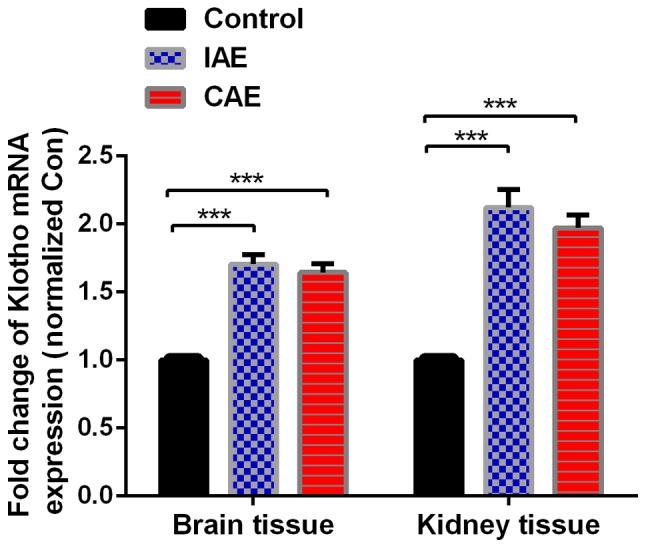
Aerobic exercise increased the mRNA expression levels of Klotho in rat brain and kidney tissues. Relative mRNA levels in rat brain and kidney tissues were detected by reverse transcription-quantitative polymerase chain reaction. GAPDH was used as an endogenous control. Data are presented as the mean + standard deviation of triplicate results (n=8 in control group, n=9 in IAE group and n=10 in CAE group). ***P<0.001. IAE, intermittent aerobic exercise; CAE, continuous aerobic exercise.
Aerobic exercise increases the Klotho protein levels in rat brain and kidney tissues
As presented in Fig. 4, following 48 weeks of aerobic training, Klotho protein levels in the rat brain and kidney tissues increased significantly (P<0.05) in the IAE and CAE groups compared with the control group. The protein levels of Klotho in the brain tissues of the IAE rats were 5.2 times more than those of the control rats, and protein levels of Klotho in the brain tissues of the CAE rats were 4.5 times more than those of the control rats. Similarly, the protein levels of Klotho in the kidney tissues of IAE rats were 5.3 times greater than those of control rats, and protein levels of Klotho in the kidney tissues of the CAE rats were 4.9-times greater than those of control rats. There was no statistically significant difference between the IAE and CAE groups (P>0.05).
Discussion
Aerobic exercise is important for improving body functions and quality of life. Previous studies have demonstrated that aerobic exercise delays aging, the retardation of cognitive functions and the decline of neurological functions (18,19). The Klotho gene is closely associated with anti-aging functions in mammals. A previous study has demonstrated that the phenotype of Klotho gene knockout mice is very similar to the features of human aging, both of which exhibit pathological manifestations such as shortened life span, atherosclerosis, osteoporosis, emphysema and reduced immune function (20).
The Klotho gene is mainly expressed in brain and kidney tissues (21,22). In animal models of kidney diseases, it has been demonstrated that the expression level of Klotho protein is reduced (23). Administration of exogenous Klotho protein attenuated renal disease and restored the function of renal tubular resorption (13). In conditional brain Klotho knockout mice, the phenotypes are also similar to the features of human aging, exhibiting neurodegeneration and reduced synapses in the hippocampus (24). Reduced expression of the Klotho gene increased the speed of nerve cell senescence and caused the degeneration of glial cells (25). In addition, it was previously demonstrated that the expression level of the Klotho gene in the brain of aged macaque monkeys was reduced. These aged macaque monkeys exhibited traits similar to those of human patients with Alzheimer's disease (26,27). It was recently demonstrated that gene therapy via Clustered Regularly Interspaced Short Palindromic Repeats technology increased the expression of Klotho, which improved the cognitive ability of experimental mice (28,29).
ROS lead to accumulation of oxidative damage to DNA, lipids and proteins in cells, which further lead to the degradation of cell functions, ultimately causing senescence. Therefore, the body needs to improve the ability to remove or resist ROS to keep healthy (30). One of the most important functions of the Klotho gene is to increase the ability of organisms to clear harmful ROS by increasing the cell functions that detoxify harmful ROS (31,32). It was previously reported that Klotho can enhance cardiac function by inhibiting ROS (31). Furthermore, previous studies have demonstrated that moderate exercise may upregulate Klotho gene expression in muscle tissues and control the production of ROS (33,34).
Based on the above findings, it was hypothesized that if aerobic exercise could increase Klotho gene expression, it would be a physiological and safe method to relieve aging and many aging-associated diseases. Consistent with these previous findings, the present experimental results also demonstrated that aerobic exercise significantly prolonged the survival time of rats and that aerobic exercise upregulated the expression of Klotho in brain and kidney tissues. The effect of IAE on Klotho expression was slightly higher than that of CAE, but the difference was not statistically significant. Previous studies have demonstrated that the Klotho protein has both membrane-bound and secretory forms in humans and mice and the latter is significantly higher than the former (35,36). However, in rats, the membrane-bound form Klotho is more prevalent, whereas the secreted form is faintly expressed (37). In the present study, expression levels of the membrane-bound Klotho protein were quantified using a rabbit polyclonal antibody specific to Klotho. The predicted molecular weight of this protein is 116 kDa. It was demonstrated that the expression of the membrane-bound Klotho protein was significantly reduced following a long period of aerobic exercise. This result suggests that the membrane-bound Klotho protein may be the active form. In addition, aerobic exercise significantly suppressed the levels of ROS in rat brain and kidney tissues. Compared with that in the IAE group, the reduction in ROS level was slightly lower in the CAE group, but the difference was not statistically significant. It has been previously reported that both types of aerobic exercise benefit the improvement of heart function and myocardial structure in myocardial infarction patients (38,39). Therefore, the aim of the present study was to test whether the two aerobic exercise methods are equally effective at delaying aging. According to the present results, both types of exercise are effective, and there is no statistically significant difference between the two methods. Therefore, any aerobic exercise method can be selected according to one's own conditions.
In conclusion, the present results results suggest that exercise could delay aging and extend life span by increasing the expression of the Klotho gene in rat brain and kidney tissues. Increased Klotho protein expression is beneficial for the elimination of ROS damage to the body, delaying aging and improving various body functions. These findings support as association among the Klotho gene, aerobic exercise and aging. As Klotho exhibits a potential anti-aging effect, promoting Klotho expression through aerobic exercise may be a novel approach for the prevention and treatment of aging and aging-related diseases. In addition, these results provide support for physical exercise being good for health.
Funding
The present study was supported by grant no. 17JK0666 from the Scientific Research Program funded by the Education Department of Shaanxi Province, grant no. 17036 from Common Projects by the Sports Bureau of Shaanxi Province, grant no. 2017GJFY36 from the National Natural Science Foundation Training Program and grant no. 2017DOC03 from the Scientific Research Foundation of Xi'an Medical University.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
NJ and KL conceived the project and designed the present study. NJ, JL, FH, YZ, WW and BL performed the experiments and data analysis, and wrote the manuscript. JL, MX and XZ performed data analysis and revised the manuscript. KL supervised the work, provided administrative support, performed data analysis and proofread the paper.
Ethics approval and consent to participate
All rat experiments were performed using protocols approved by the Institutional Ethics Committee on Animal Use of Xi'an Medical University (Xi'an, China).