- Journal List
- eLife
- PMC5957528

Boosting ATM activity alleviates aging and extends lifespan in a mouse model of progeria
Minxian Qian
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Zuojun Liu
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Linyuan Peng
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Xiaolong Tang
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Fanbiao Meng
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Ying Ao
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Mingyan Zhou
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Ming Wang
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
4South China Institute for Stem Cell Biology and Regenerative Medicine, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China
Xinyue Cao
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Baoming Qin
4South China Institute for Stem Cell Biology and Regenerative Medicine, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou, China
Zimei Wang
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Zhongjun Zhou
5School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong, China
Guangming Wang
6East Hospital, Tongji University School of Medicine, Shanghai, China
7Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China
Zhengliang Gao
7Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China
8Advanced Institute of Translational Medicine, Tongji University, Shanghai, China
Baohua Liu
1Guangdong Key Laboratory of Genome Stability and Human Disease Prevention, Shenzhen University Health Science Center, Shenzhen, China
2Medical Research Center, Shenzhen University Health Science Center, Shenzhen, China
3Department of Biochemistry and Molecular Biology, Shenzhen University Health Science Center, Shenzhen, China
Associated Data
- Supplementary Materials
- Figure 1—source data 1: Statistical analysis for SA-β-Gal positive staining.DOI: 10.7554/eLife.34836.006Figure 1—source data 2: Statistical analysis for EdU positive staining.DOI: 10.7554/eLife.34836.007Figure 2—source data 1: Differently expressed mRNA profiles of Atm-/- MEF cells.elife-34836-fig2-data1.xlsx (1.3M)DOI: 10.7554/eLife.34836.011Figure 2—source data 2: Differentially expressed Metabolites in Atm KO MEFs.DOI: 10.7554/eLife.34836.012Figure 2—source data 3: Differently expressed mRNA profiles of Sirt6-/- MEF cells.elife-34836-fig2-data3.xlsx (1.3M)DOI: 10.7554/eLife.34836.013Figure 5—source data 1: Lifespan analysis of chloroquine's effect on nematodes.DOI: 10.7554/eLife.34836.021Figure 5—source data 2: Lifespan analysis of chloroquine's effect on mice.DOI: 10.7554/eLife.34836.022
- Data Availability Statement
Sequencing data have been deposited in GEO under accession code {"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280.
The following dataset was generated:
Qian MLiu B2018Boosting ATM activity alleviates ageing and extends lifespan in a mouse model of progeria.https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc={"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280Publicly available at the NCBI Gene Expression Omnibus (accession no: {"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280).
Abstract
DNA damage accumulates with age (Lombard et al., 2005). However, whether and how robust DNA repair machinery promotes longevity is elusive. Here, we demonstrate that ATM-centered DNA damage response (DDR) progressively declines with senescence and age, while low dose of chloroquine (CQ) activates ATM, promotes DNA damage clearance, rescues age-related metabolic shift, and prolongs replicative lifespan. Molecularly, ATM phosphorylates SIRT6 deacetylase and thus prevents MDM2-mediated ubiquitination and proteasomal degradation. Extra copies of Sirt6 extend lifespan in Atm-/- mice, with restored metabolic homeostasis. Moreover, the treatment with CQ remarkably extends lifespan of Caenorhabditis elegans, but not the ATM-1 mutants. In a progeria mouse model with low DNA repair capacity, long-term administration of CQ ameliorates premature aging features and extends lifespan. Thus, our data highlights a pro-longevity role of ATM, for the first time establishing direct causal links between robust DNA repair machinery and longevity, and providing therapeutic strategy for progeria and age-related metabolic diseases.
eLife digest
As cells live and divide, their genetic material gets damaged. The DNA damage response is a network of proteins that monitor, recognize and fix the damage, which is also called DNA lesions. For example, an enzyme called ATM senses when DNA is broken and then begins a process that will get it repaired, while another enzyme known as SIRT6 participates in the actual mending process.
When organisms get older, the repair machinery becomes less efficient, and the number of DNA lesions and errors increases. The accumulation of DNA damage may cause the ‘symptoms’ of old age – from cancer, to wrinkles and the slowing down of the body’s chemical processes. In fact, individuals with defective ATMs (who thus struggle to repair their DNA) age abnormally fast; conversely, SIRT6 promotes longevity. If declining repair mechanisms cause aging, would boosting the DNA damage response slow down this process?
Chloroquine is a drug used to combat malaria, but it can also enhance the activity of ATM without damaging DNA. Qian, Liu et al. show that chloroquine helps cells remove broken DNA and keep dividing for longer. In animals, the drug increases the lifespan of worms and prolongs the lives of mice who have mutations that make them age quicker.
Qian, Liu et al. also demonstrate that ATM works by chemically altering the pro-longevity enzyme SIRT6. These changes make SIRT6 more stable, and keep it safe from cellular processes that destroy it. In addition, mice that are genetically engineered to lack ATM can survive for longer if they also produce extra SIRT6. These experiments show that enhancing the DNA damage response can slow down aging, thus linking the DNA repair machinery to longevity.
Progeria is a group of rare genetic conditions with inefficient DNA repair; people with progeria age fast and die young. The results by Qian, Liu et al., if confirmed in humans, could provide a new way of treating these diseases.
Introduction
A variety of metabolic insults can generate DNA lesions in mammalian cells, which, if incorrectly repaired, can lead to somatic mutations and cell transformation (Vijg, 2014). If unrepaired, such lesions can accumulate and constantly activate the DNA damage response (DDR), a unique feature and mechanism of senescence (Halliwell and Whiteman, 2004; Tanaka et al., 2006). Ataxia telangiectasia mutated (ATM), a serine/threonine protein kinase, is one of the key regulators of DDR (Guleria and Chandna, 2016). Upon DNA damage, self-activated ATM phosphorylates downstream transducers and effectors, promoting DNA repair (Bakkenist and Kastan, 2003; Paull, 2015). H2AX is a well-documented phosphorylation target of ATM; phosphorylated H2AX at S139 (γH2AX) is widely recognized as a hallmark of DNA damage (Burma et al., 2001). Accompanied by decline in DNA repair function, γH2AX-enriched DNA damage foci accumulate in senescent cells and in tissues from aged animals (Maslov and Vijg, 2009), supporting causal links between defective DDR and aging. In human fibroblasts, a dramatic decline of homologous recombination (HR) efficiency, attributable to defective recruitment of Rad51, has been observed (Li et al., 2016). Similar defects in HR also have been observed in Hutchinson-Gilford progeria syndrome (HGPS), which is predominantly caused by a LMNA C1024T mutation (Liu et al., 2005).
In addition to DNA damage accumulation, inherited loss-of-function mutations in essential components of DNA repair machinery accelerate aging in humans and mice (Hoeijmakers, 2009b). Patients suffering from ataxia telangiectasia (A-T) develop prominent aging features in their second decades (Boder and Sedgwick, 1958; Shiloh and Lederman, 2017). Werner syndrome, Bloom’s syndrome and Rothmund-Thomson syndrome are all progeria syndromes caused by mutations of genes that directly regulate DNA repair (Balajee et al., 1999; Cooper et al., 2000; Lebel et al., 1999; Li and Comai, 2000). Homozygous disruption of Atm in mice causes many premature aging features of A-T, such as growth retardation, infertility, neurodegeneration, immunodeficiency and cancer predisposition (Barlow et al., 1996). Mouse models deficient in DNA repair factors, including DNA-PKcs, Ku70, Ku80, DNA ligase IV, Artemis or Ercc1 etc., phenocopy premature aging features (Hasty, 2005; Hoeijmakers, 2009a), supporting the suggestion that defects in DNA repair accelerate aging. However, whether and how robust DNA repair machinery promotes longevity is poorly understood.
Metabolic disturbance is another antagonistic hallmark of aging (López-Otín et al., 2013). Although DNA repair deficiency is implicated in aging and age-related diseases including metabolic disorders (López-Otín et al., 2016; Shimizu et al., 2014), the mechanistic link between decreased DNA repair machinery and metabolic reprogramming during aging is poorly understood. Notably, in response to oxidative stress, ATM phosphorylates Hsp27, shifting glucose metabolism from glycolysis to the pentose phosphate pathway (PPP) (Cosentino et al., 2011; Krüger and Ralser, 2011). Inactivating ATM enhances glucose and glutamine consumption by inhibiting P53 and upregulating c-MYC (Aird et al., 2015). However, the role of ATM in age-onset metabolic disturbances is as yet unclear.
Here, we identified a progressive decline in ATM-centered DNA repair machinery during aging, along with shunted glucose metabolism to glycolysis. DNA damage-free activation of ATM by chloroquine (CQ) promotes DNA damage clearance, rescues age-related metabolic shift, and alleviates cellular senescence. Mechanistically, ATM phosphorylates and stabilizes pro-longevity protein SIRT6. Extra copies of Sirt6 attenuate metabolic abnormality and extend lifespan in Atm-/- mice. Importantly, long-term treatment of CQ restores metabolic reprogramming and extends the lifespan of nematodes and a progeria mouse model.
Results
ATM activation alleviates replicative senescence
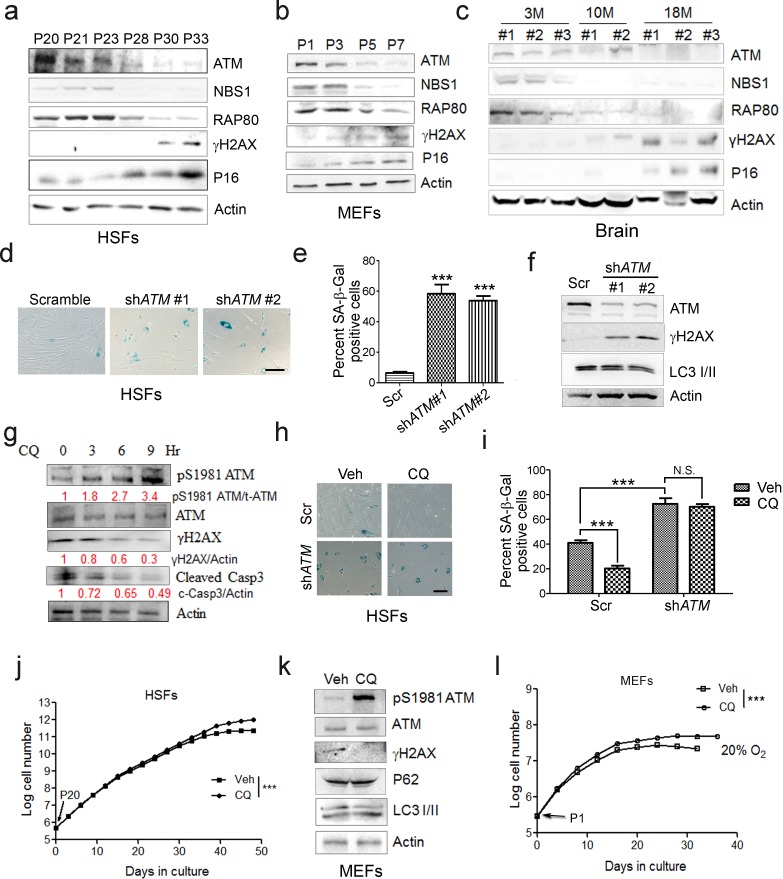
In searching for genes/pathways that drive senescence, we employed human primary endothelial cells, which underwent replicative senescence at passage 21, with increased p21 expression and β-galactosidase activity (Figure 1—figure supplement 1a–b). By RNAseq analysis, a gradual decline of ATM-centered DNA repair machinery was identified (Figure 1—figure supplement 1c–e). Western blotting analysis confirmed progressively downregulated protein levels of ATM and its downstream target NBS1 and RAP80 in senescent human skin fibroblasts (HSFs) (Figure 1a). Mouse embryonic fibroblasts (MEFs) with limited growth capacity and senescent phenotypes when cultured in vitro (Parrinello et al., 2003; Samper et al., 2003; Sherr and DePinho, 2000), and brain tissues from aged mice also showed progressive decline of ATM, NBS1, and RAP80 (Figure 1b–c). Concomitantly, upregulation of γH2AX, indicating accumulated DNA damage, and an increase in p16Ink4a were observed in senescent HSFs, MEFs, and aged brain tissues (Figure 1a–c). Knocking down ATM via shRNA accelerated senescence in HSFs, evidenced by increased β-galactosidase activity (Figure 1d–e), enlarged morphology (Figure 1—figure supplement 2a), accumulated γH2AX (Figure 1f), and reduced cell proliferation (Figure 1—figure supplement 2b). These data indicate that ATM decline retards DDR and drives senescence.
(a) Immunoblots showing protein levels of ATM, NBS1, and RAP80 in human skin fibroblasts (HSFs). A gradually increased level of p16 indicates cellular senescence, while elevated γH2AX level indicates accumulated DNA damage. (b) Immunoblots showing protein levels of ATM, NBS1, and RAP80 in mouse embryonic fibroblasts (MEFs). (c) Immunoblots showing protein levels of ATM, NBS1, and RAP80 in brain tissues isolated from 3-, 10-, and 18-month-old male mice. (d) SA-β-Gal staining in HSFs treated with sh-ATM or scramble shRNA. Scale bar, 100 µm. (e) Quantification of SA-β-Gal-positive staining of (d) from five views randomly captured for each group. Data represent means ± SEM. ***p<0.001. (f) Immunoblots showing increased γH2AX and unaffected LC3I/II in HSFs treated with sh-ATM or scramble shRNA. (g) Immunoblots showing protein levels of pS1981 ATM, γH2AX, and cleaved caspase-3 in HSFs treated with 10 μM of CQ for indicated time. (h) SA-β-Gal staining in HSFs expressing either scramble or ATM shRNA treated with 1 μM CQ or DMSO (12 hr). Scale bar, 100 µm. (i) Quantification of SA-β-Gal-positive staining of (h) from five views randomly captured for each group. Data represent means ± SEM. ***p<0.001; ‘N.S.’ indicates no significant difference. (j) HSFs at passage 20 were continuously cultured with 1 μM CQ or DMSO, and cell number was calculated at each passage. Data represent means ± SEM. ***p<0.01. (k) Immunoblots showing protein levels of γH2AX, p62, and LC3 in MEFs treated with 1 μM CQ or DMSO. Note that CQ had little effect on the expression levels of p62 and LC3. (l) MEFs at passage one were continuously cultured in 20% O2 with 1 μM CQ or DMSO, and cell number was determined at each passage. Data represent means ± SEM. ***p<0.01.
Figure 1—source data 1.
Statistical analysis for SA-β-Gal positive staining.Figure 1—source data 2.
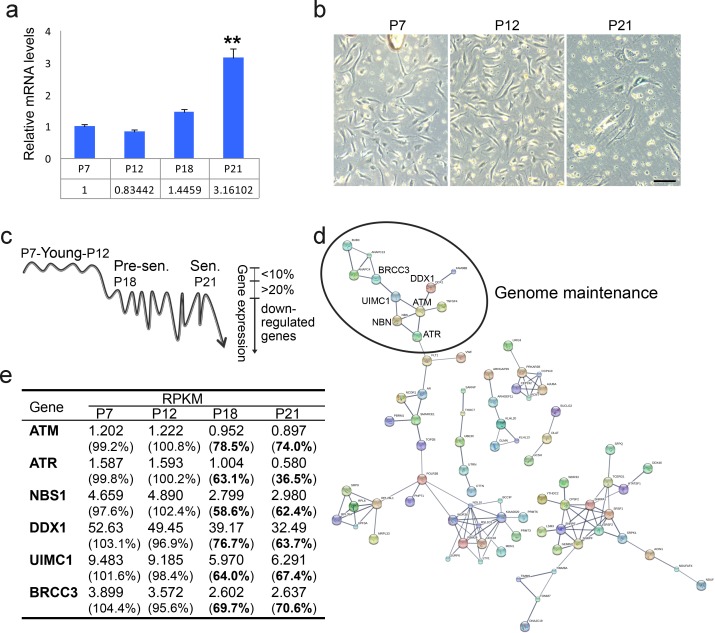
Statistical analysis for EdU positive staining.Figure 1—figure supplement 1.
(a) Real-time PCR analysis showing progressively elevated mRNA level of p21 in continuously cultured human endothelial cells (HUVEC). **p<0.01. (b) SA-β-Gal staining of HUVEC cells at indicated passages. Scale bar, 100 µm. (c) HUVEC cells at P21, P18, P12, and P7 were subjected to transcriptome analysis. A minimum average rpkm value of 1.0 and maximum 10% fluctuation in young cells (P7 Vs P12) was set as the threshold. Genes were downregulated by more than 20% in pre-senescent, and senescent cells compared with young cells (P21/P18 Vs P12/P7) were selected. (d) Pathway analysis of genes identified in (c) by STRING v10. (e) Downregulation of ATM-related DNA repair genes during senescence.
Figure 1—figure supplement 2.
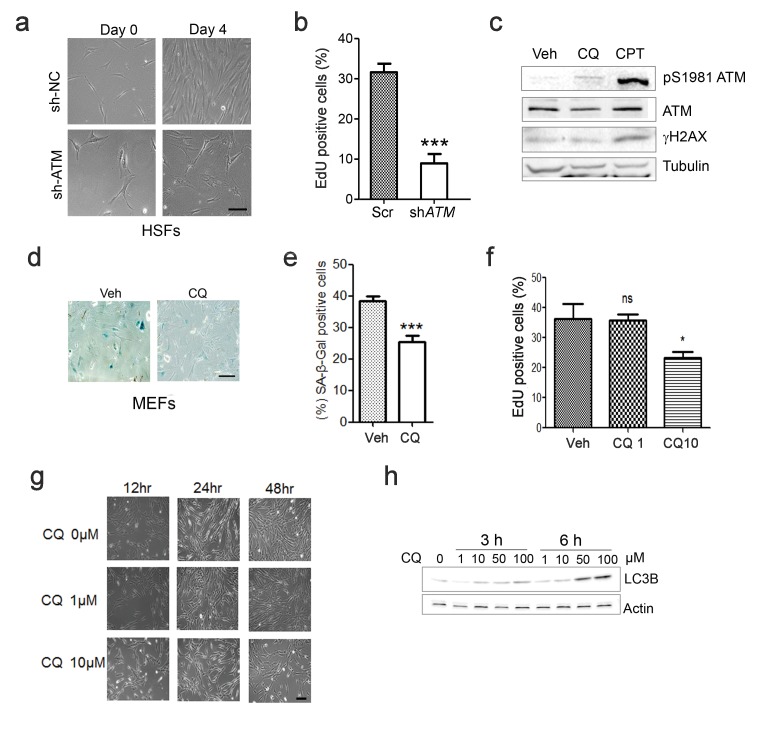
(a) Representative images showing cells treated with Scramble (sh-NC) or sh-ATM. (b) Percent EdU-positive cells in sh-NC or sh-ATM treated HSFs. Views were randomly captured and at least 100 cells were included in each group. Data represent means ± SEM. ***p<0.001. (c) Immunoblots showing protein levels of pS1981 ATM and γH2AX in HSFs treated with 10 μM chloroquine (CQ) or 0.4 μM CPT (4 hr). Note that CQ activated ATM (pS1981) without increasing γH2AX, while CPT activated ATM accompanied by increased γH2AX. (d) SA-β-Gal staining in primary MEFs treated with 1 μM CQ or DMSO. Scale bar, 100 µm. (e) Quantification of SA-β-Gal-positive staining of (d) from five views randomly captured for each group. Data represent means ± SEM. ***p<0.001. (f) Percent EdU-positive cells in HSFs treated with DMSO, 1 μM or 10 μM CQ. Views were randomly captured and at least 100 cells were included in each group. Data represent means ± SEM. ***p<0.001. (g) Representative images showing proliferative HSFs treated with different doses of CQ for the indicated time points. (h) Immunoblots showing LC3B levels in HSFs treated with indicated dose of CQ for indicated period of time.
Other than DNA damage, ATM is activated by chloroquine (CQ), an antimalarial drug that modulates chromatin confirmation (Bakkenist and Kastan, 2003). We confirmed that a low dose of CQ increased the level of pS1981 auto-phosphorylation of ATM but not γH2AX (Figure 1—figure supplement 2c). We then investigated whether activating ATM by CQ can ameliorate senescence. As shown, the CQ treatment activated ATM (pS1981), promoted clearance of DNA damage (γH2AX), and inhibited apoptosis (cleaved Casp3) in HSFs (Figure 1g). Also, the CQ treatment suppressed β-galactosidase activity, which was abrogated if ATM was knocked down (Figure 1h–i). Importantly, CQ treatment extended the replicative lifespan of HSFs (Figure 1j). Likewise, CQ treatment activated Atm, cleared up accumulated DNA damage, suppressed β-galactosidase activity (Figure 1k and Figure 1—figure supplement 2d–e), and prolonged replicative lifespan in MEFs (Figure 1l). Although both 10 μM and 1 μM of CQ activated ATM, a dose-dependent toxicity assay showed that 1 μM is suitable for long-term treatment (Figure 1—figure supplement 2f–g). Of note, ATM KD or low dose of CQ applied in this study had little effect on basal autophagic activity (Figure 1f,j and Figure 1—figure supplement 2g). Collectively, CQ activates ATM to alleviate replicative senescence.
An ATM-SIRT6 axis underlies age-associated metabolic reprogramming
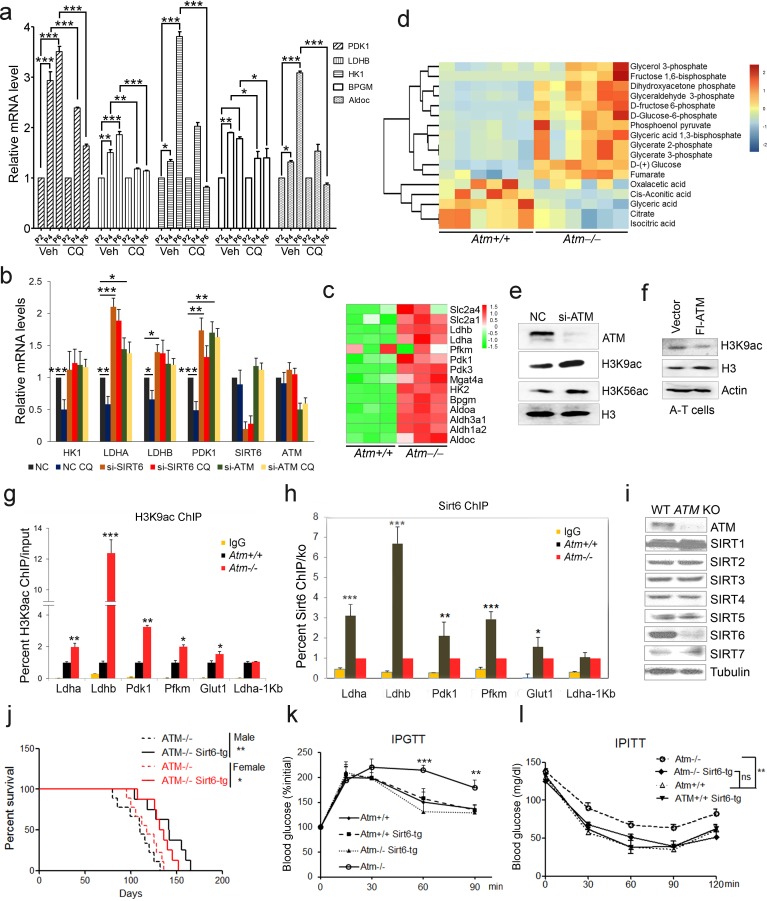
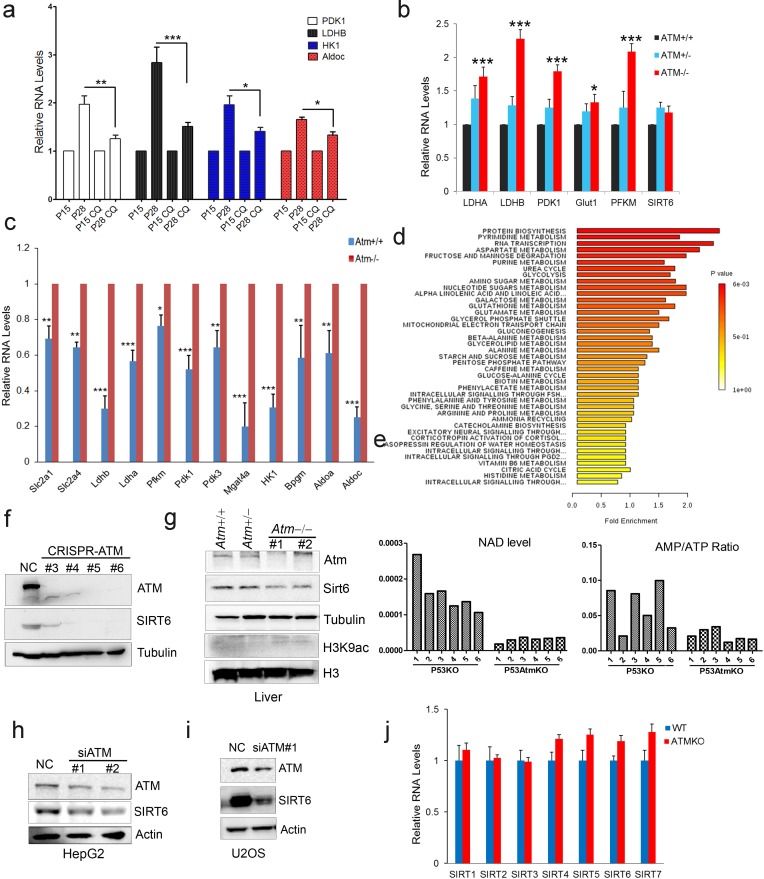
A-T patients lacking functional ATM display features of premature aging, accompanied by insulin resistance and glucose intolerance (Bar et al., 1978; Espach et al., 2015). Senescent cells exhibit impaired mitochondrial respiration, but enhanced glycolysis producing more lactate (Hagen et al., 1997; Lenaz et al., 2000). As such, we wondered whether ATM decline triggers an age-associated metabolic shift. Levels of glycolytic genes LDHB and PDK1 were dramatically increased in senescent MEFs and HSFs (Figure 2a and Figure 2—figure supplement 1a), and in liver tissues from Atm-/- mice (Figure 2—figure supplement 1b). Significantly, activating ATM via CQ suppressed senescence-associated glycolysis (Figure 2a and Figure 2—figure supplement 1a). Similarly, the inhibitory effect on glycolysis was diminished when ATM was depleted in HepG2 cells (Figure 2b). These data suggest a role for ATM in inhibiting glycolysis.
(a) Quantitative RT-PCR analysis of mRNA levels of indicated glycolytic genes in different passages of MEFs with or without treatment of CQ. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. (b) Quantitative RT-PCR analysis of mRNA levels of indicated glycolytic genes in Scramble (NC), si-SIRT6 or si-ATM HepG2 cells incubated with or without CQ (10 μM, 6 hr). Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. (c) Heatmap representation of RNA-Seq data ({"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280) showing relative changes of glycolytic genes in Atm-/- MEF cells. The transcript levels are qualified in reads per kilobase of exon per million mapped sequence reads (RPKM), which is a normalized measure of exonic read density. Red and green indicate up- and downregulation, respectively. (d) Heatmap showing relative levels of metabolites in Atm+/+ and Atm-/- MEF cells of p53 null background, analyzed by LC-MS. Red and blue indicate up- and downregulation, respectively. (e) Immunoblots showing protein levels of H3K9ac and H3K56ac in ATM-deficient HepG2 cells. (f) Immunoblots showing levels of H3K9ac in A-T cells reconstituted with Flag-ATM. (g) ChIP analysis showing enrichment of H3K9ac at the promoter regions of indicated genes in Atm+/+ and Atm-/- MEFs. Data represent means ± SEM of three independent experiments. *p<0.05, **p<0.01, ***p<0.001. (h) ChIP analysis showing enrichment of Sirt6 at the promoter regions of indicated genes in Atm+/+ and Atm-/- MEFs. Data represent means ± SEM of six independent experiments. *p<0.05, **p<0.01, ***p<0.001. (i) Immunoblots showing protein levels of sirtuins in wild-type (WT) and ATM knockout (KO) HEK293 cells. (j) Kaplan-Meier survival of Atm-/- and Atm-/-;Sirt6-Tg male (n = 11 in each group) and female (n = 9 in each group) mice. **p<0.01. (k) Results of glucose tolerance tests in Atm+/+, Atm-/-, and Atm-/-;Sirt6-Tg mice. Data represent means ± SEM, n = 6. **p<0.01, ***p<0.001. (l) Results of insulin tolerance tests in Atm+/+, Atm-/-, and Atm-/-;Sirt6-Tg mice. Data represent means ± SEM, n = 6. **p<0.01. ‘ns’ indicates no significant difference.
Figure 2—source data 1.
Differently expressed mRNA profiles of Atm-/- MEF cells.Figure 2—source data 2.
Differentially expressed Metabolites in Atm KO MEFs.Figure 2—source data 3.
Differently expressed mRNA profiles of Sirt6-/- MEF cells.Figure 2—figure supplement 1.
(a) Quantitative RT-PCR analysis of mRNA levels of indicated glycolytic genes in different passages of HSFs with or without treatment of CQ. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. (b) Quantitative RT-PCR analysis of mRNA levels of glycolytic genes in liver tissues from Atm+/+, Atm+/-, and Atm-/- mice. Data represent mean ± SEM. Atm-/- Vs Atm+/+, *p<0.05, ***p<0.001. (c) Quantitative RT-PCR analysis of mRNA levels of glycolytic genes to validate the RNA-seq data set of Atm+/+ and Atm-/- MEFs. Data represent means ± SEM of six independent experiments. *p<0.05, **p<0.01, ***p<0.001. (d) Metabolic pathway enrichment in Atm null MEFs compared with wild-types. (e) AMP/ATP ratio and NAD + levels in wild-type and Atm null MEFs. (f) Immunoblots showing ATM and SIRT6 protein levels in indicated clones of ATM KO HEK293 cells generated by the CRISPR/Cas9 system. (g) Immunoblots showing protein levels of Sirt6 and H3K9ac in liver tissues from 4-month-old Atm+/+, Atm+/-, and Atm-/- mice. (h–i) Immunoblots showing SIRT6 levels in si-NC and si-ATM treated HepG2 and U2OS cells. (j) Quantitative RT-PCR analysis of sirtuin mRNA levels in wild-type and ATM KO HEK293 cells. Data represent means ± SEM.
Figure 2—figure supplement 2.
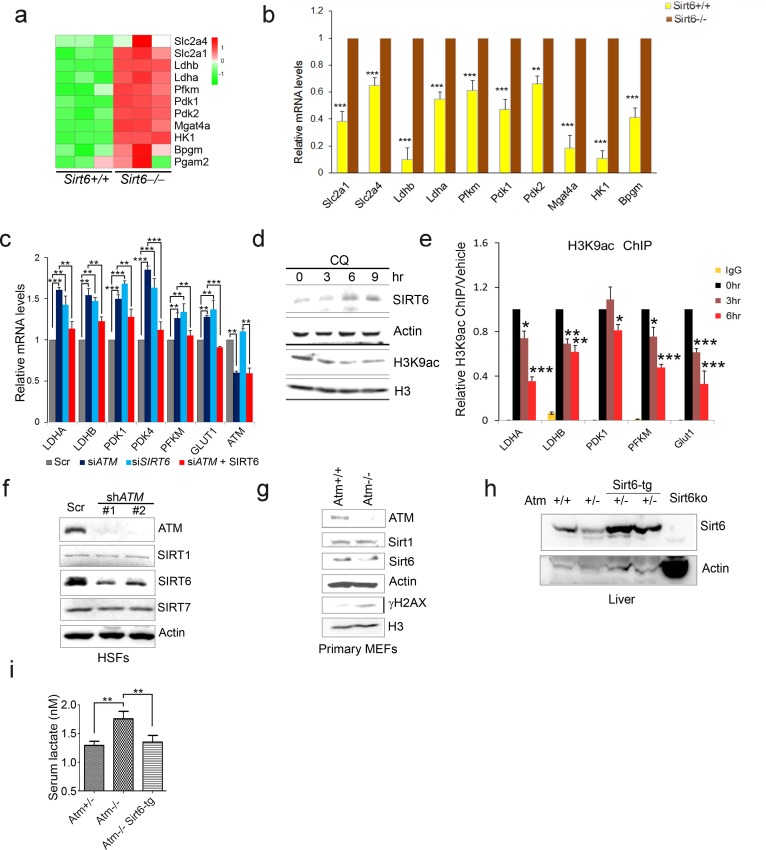
(a) Heatmap representation of RNA-Seq data showing relative changes of glycolytic genes in Sirt6-/- MEF cells. Red and green indicate up- and downregulation, respectively. (b) Quantitative RT-PCR analysis of mRNA levels of glycolytic genes to validate the RNA-seq data set of Sirt6+/+ and Sirt6-/- MEFs. Data represent means ± SEM of six independent experiments. **p<0.01, ***p<0.001. (c) Quantitative RT-PCR analysis of mRNA levels of indicated glycolytic genes in HepG2 cells transfected with Scramble, si-ATM, si-SIRT6, or si-ATM plus Flag-SIRT6. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. (d) Immunoblots showing protein levels of SIRT6 and H3K9ac in HepG2 cells treated with 10 μM CQ for indicated periods of time. (e) ChIP analysis showing enrichment of H3K9ac at promoter regions of glycolytic genes in HepG2 cells treated with 10 μM CQ for indicated periods of time. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. (f) Immunoblots showing SIRT1, SIRT6, and SIRT7 protein levels in ATM knockdown (KD) or control HSFs. (g) Immunoblots showing protein levels of Atm, Sirt1, Sirt6 and γH2AX levels in Atm+/+ and Atm-/- primary MEF cells. (h) Immunoblots showing Sirt6 expression in liver tissues of Sirt6-tg mice. (i) Serum lactate levels in 3-month-old Atm+/+, Atm-/- and Atm-/-;Sirt6-tg male mice. Data represent mean ± SEM of five to six animals/group. *p<0.05.
To examine how ATM regulates glycolysis, we performed RNA-Seq in Atm-/- MEF cells, and revealed a significant upregulation of glycolytic pathways (Figure 2c, and Figure 2—source data 1). Specific genes were validated by q-PCR (Figure 2—figure supplement 1c). As p53 is critical in glycolysis (Kruiswijk et al., 2015; Schwartzenberg-Bar-Yoseph et al., 2004), we further analyzed metabolomics of Atm-/- and control MEFs in p53 null background. As shown, the metabolic profile exhibited a clear shift, i.e. mitochondrial electron transport chain and intermediates of TCA cycle were reduced, while intermediates of glycolysis were elevated (Figure 2d, Figure 2—figure supplement 1d–e and Figure 2—source data 2). The data suggest that ATM deficiency enhances anaerobic glycolysis in a p53-independent manner.
Sirt6 deacylase is able to shunt energy metabolism away from anaerobic glycolysis to the TCA cycle via H3K9ac-mediated local chromatin remodeling (Sebastián et al., 2012; Zhong et al., 2010). We noted that the level of H3K9ac was enhanced in cells depleted ATM (Figure 2e). Re-expressed ATM in A-T cells suppressed H3K9ac level (Figure 2f). ChIP analysis showed that H3K9ac was enriched at the promoter regions of glycolytic genes in Atm-/- cells (Figure 2g), where the relative occupancy of SIRT6 was abolished (Figure 2h). Consistent with increased H3K9ac, SIRT6 protein level was dramatically downregulated in Atm-/- mouse livers, and ATM-deficient HepG2, U2OS and HEK293 cells (Figure 2—figure supplement 1f–i). In contrast, protein levels of other sirtuins were not much affected in ATM KO HEK293 cells (Figure 2i), and mRNA levels of all sirtuins remained unchanged (Figure 2—figure supplement 1j). Moreover, transcriptomic analysis and q-PCR data illustrated that Sirt6 depletion upregulated a similar cluster of genes essential for glycolysis (Figure 2—figure supplement 2a–b and Figure 2—source data 3). More importantly, the hyper-activated glycolytic pathway caused by ATM deficiency was completely restored by ectopic SIRT6 in HepG2 cells (Figure 2—figure supplement 2c). The CQ treatment upregulated SIRT6 level and reduced H3K9ac level, especially at the regulatory regions of glycolytic genes (Figure 2—figure supplement 2d–e). Knocking down SIRT6 abolished the inhibitory effect of CQ on glycolysis (Figure 2b). Additionally, ATM depletion in HEK293 cells, HSFs, and MEFs, significantly downregulated SIRT6 protein level, with little effect on SIRT1 or SIRT7 (Figure 2i and Figure 2—figure supplement 2f–g). Thus, these data suggest that ATM decline triggers an age-associated metabolic shift via SIRT6-mediated chromatin remodeling.
Other than metabolic abnormality, depleting Sirt6 leads to premature aging features and shortened lifespan (Mostoslavsky et al., 2006a), whereas extra copies of Sirt6 promote longevity in male mice (Kanfi et al., 2012). Given that Sirt6 was destabilized in Atm null mice, we wondered whether the Sirt6 transgene could rescue premature aging phenotypes and shortened lifespan in Atm-/- mice. To this end, we generated Sirt6 transgenic mice by microinjection, and bred them with Atm-/- mice. The overexpression of Sirt6 was demonstrated by western blotting (Figure 2—figure supplement 2h). Significantly, ectopic Sirt6 restored the elevation of serum lactate, and extended lifespan of Atm-/- mice of both genders (Figure 2j and Figure 2—figure supplement 2i). Importantly, Atm-/-;Sirt6-tg mice exhibited improved glucose tolerance and decreased insulin resistance (Figure 2k–l). Given that little difference was observed in glucose metabolism between young wild-type (WT) and Sirt6-transgenic mice (Kanfi et al., 2012), these data suggest a contributory role of the Atm-Sirt6 axis in the age-associated metabolic reprogramming.
ATM phosphorylates and stabilizes SIRT6
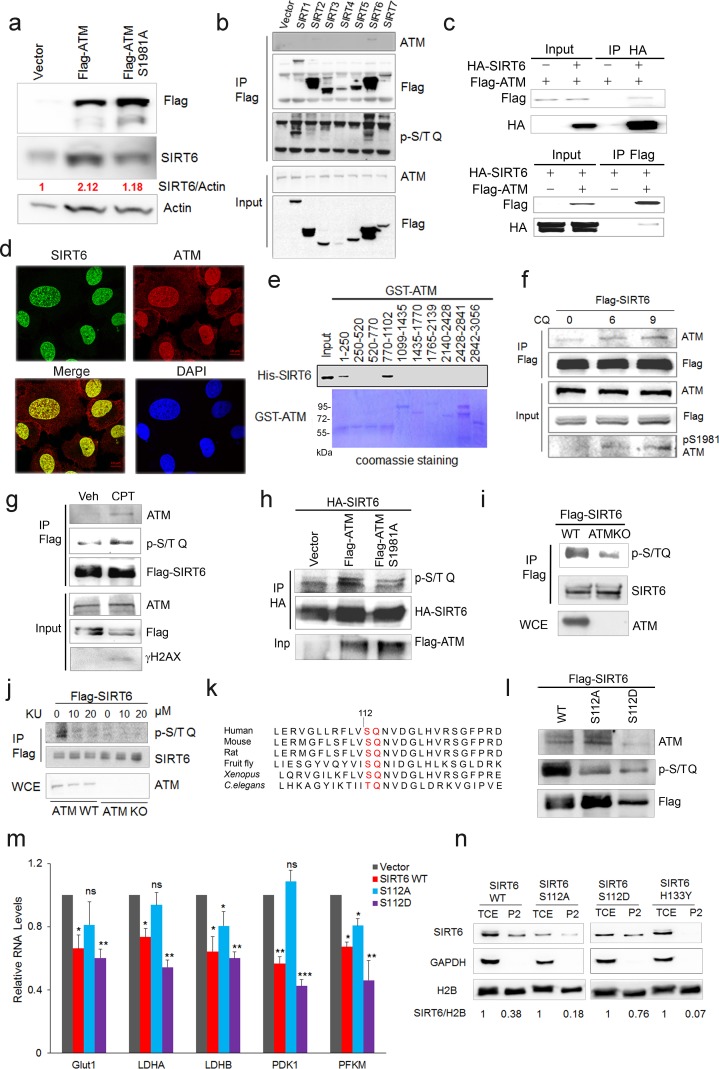
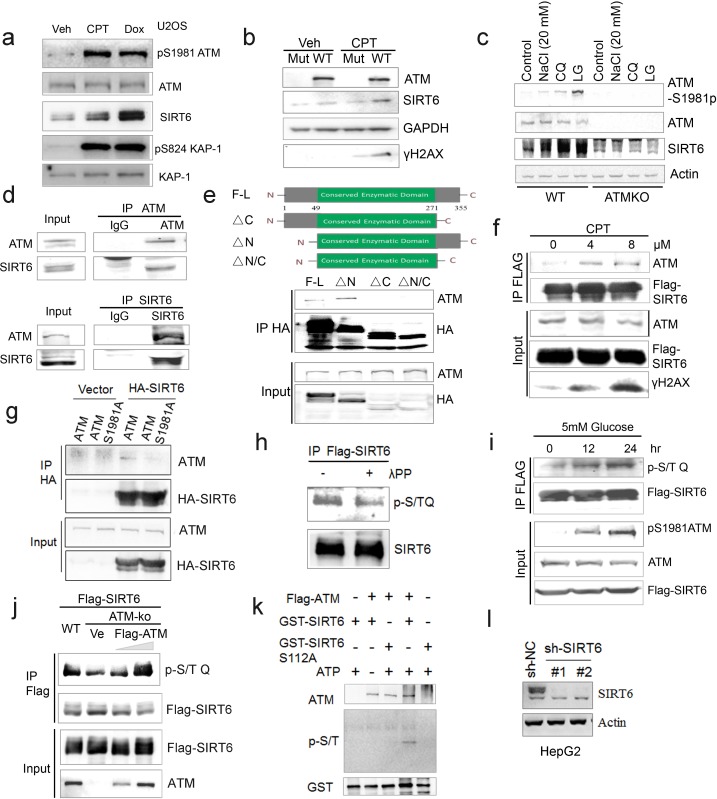
Next, we examined how ATM regulates SIRT6. Significantly, overexpression of ATM increased SIRT6 level, but this was abolished when ATM was S1981A-mutated to block dimeric ATM dissociation (Bakkenist and Kastan, 2003; Berkovich et al., 2007) (Figure 3a). Moreover, in addition to CQ, hypotonic buffer (20 mM NaCl), low glucose (LG), DNA-damaging agent camptothecin (CPT), and doxorubicin (Dox) all activated ATM and concomitantly increased SIRT6 protein level (Figure 3—figure supplement 1a–c), which was abrogated in ATM-depleted cells (Figure 3—figure supplement 1b–c). These data implicate a direct regulation of SIRT6 stability by ATM kinase activity. To confirm this, we first performed co-immunoprecipitation (Co-IP) in cells transfected with various FLAG-sirtuins. Interestingly, ATM was predominantly associated with SIRT6 among seven sirtuins (Figure 3b). The interaction was further confirmed at both ectopic and endogenous levels (Figure 3c and Figure 3—figure supplement 1d). Immunofluorescence microscopy showed co-localization of SIRT6 and ATM protein in the nucleus (Figure 3d). A domain mapping experiment indicated that the C-terminal domain was required for SIRT6 binding to ATM (Figure 3—figure supplement 1e). To determine whether ATM physically binds to SIRT6, 10 consecutive recombinant GST-ATM proteins were obtained and the binding to purified His-SIRT6 was analyzed. As shown (Figure 3e), His-SIRT6 bound predominantly to GST-ATM-4 (residues 770–1102) and relatively weakly to GST-ATM-1 (residues 1–250); both belong to the N-terminal HEAT repeat domain of ATM.
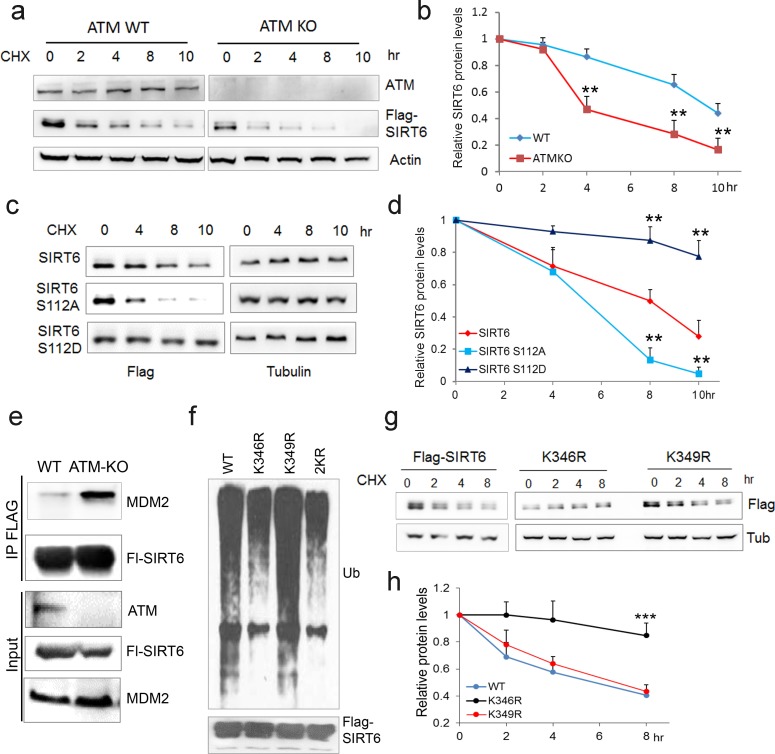
(a) Immunoblots showing protein levels of SIRT6 in HEK293 cells expressing Flag-ATM or Flag-ATM S1981A. (b) Immunoblots showing endogenous ATM and p-S/TQ motif in anti-Flag immunoprecipitates in HEK293 cells transfected with empty vector or Flag-sirtuins. (c) Immunoblots showing Flag-ATM and HA-SIRT6 in anti-HA (upper) or anti-Flag (lower) immunoprecipitates in HEK293 cells transfected with indicated constructs. (d) Representative photos of immunofluorescence staining of SIRT6 and ATM in U2OS cells, showing co-localization in the nucleus. Scale bar, 50 µm. (e) GST pull-down assay showing bacterially expressed His-SIRT6 predominantly bound to GST-ATM fragment 4 (770–1102), the N-terminal HEAT-repeat of ATM. (f) Immunoblots showing the increased binding capacity of ATM and SIRT6 under the treatment of (10 µM) CQ for the indicated time. (g) Immunoblots showing ATM and p-S/TQ in anti-Flag immunoprecipitates in HEK293 cells expressing Flag-SIRT6 treated with CPT (0.4 µM) or DMSO. (h) Immunoblots showing level of p-S/TQ SIRT6 in HEK293 cells co-transfected with HA-SIRT6 and Flag-ATM, Flag-ATM S1981A, or empty vector. (i) Immunoblots showing p-S/T Q SIRT6 in WT or ATM KO HEK293 cells. (j) Immunoblots showing p-S/T Q level of SIRT6 in ATM WT or KO HEK293 cells treated with DMSO and KU55933 (10 or 20 µM, 2 hr). (k) Alignment of protein sequence of human SIRT6 and orthologues in mouse, rat, fruit fly, Xenopus, and C. elegans. A conserved S112 Q113 motif was highlighted. (l) Immunoblots showing p-S/T Q level of Flag-SIRT6, Flag-SIRT6 S112A, or Flag-SIRT6 S112D in HEK293 cells. (m) Quantitative RT-PCR analysis of mRNA levels of indicated glycolytic genes in sh-SIRT6 HepG2 cells re-expressing SIRT6, SIRT6 S112A, or 112D mutation. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. ‘ns’ indicates no significant difference. (n) Immunoblots showing SIRT6 protein level in total cell extract (TCE) and chromatin-enriched fractions (P2). Densitometry analysis was performed to determine the relative ratio of SIRT6/H2B within chromatin fractions.
Figure 3—figure supplement 1.
(a) Immunoblots showing a significant increase in SIRT6 protein level, pS1981ATM, and pS824KAP-1 in the presence of DNA damage reagents-CPT and Dox in U2OS cells. (b) Immunoblots showing Sirt6 protein level in immortalized wild-type and Atm null MEFs in response to 0.4 μM CPT treatment. (c) Immunoblots showing pS1981-ATM and SIRT6 levels in wild-type and ATM KO HEK293 cells treated with CQ, low glucose (LG), and hypotonic swelling (20 mM NaCl). (d) Immunoblots of indicated immunoprecipitates showing the interaction between endogenous ATM and SIRT6 in HepG2 cells. (e) Immunoblots showing interaction between ATM and truncated fragments of SIRT6 in HEK293 cells. F-L, full-length of SIRT6, ΔC, C-terminal deletion, ΔN, N-terminal deletion, ΔN-C, N-and C-terminal truncation. (f) Immunoblots showing ATM protein in anti-Flag immunoprecipitates in HEK293 cells expressing Flag-SIRT6, treated with 0 μM, 4 μM, or 8 μM of CPT for 1 hr. (g) Immunoblots showing ATM protein in anti-HA immunoprecipitates in HEK293 cells expressing Flag-ATM or Flag-ATM S1981A, as well as empty vector or HA-SIRT6. (h) Immunoblots showing p-S/T Q of Flag-SIRT6 in HepG2 cells treated with 5 mM of glucose for indicated time. (i) Immunoblots showing p-S/T Q of Flag-SIRT6 in HEK293 cells, treated with or without λPP (30 min). (j) Immunoblots showing p-S/T Q of SIRT6 in WT and ATM KO HEK293 cells, transfected with empty vector, 2 μg Flag-ATM, or 6 μg Flag-ATM. (k) Immunoblots showing p-S/T Q of GST-SIRT6 after incubation with Flag-ATM purified from CPT-treated HEK293 cells. (l) Immunoblots showing SIRT6 protein levels in HepG2 cells with lentiviral infection containing sh-NC or sh-SIRT6.
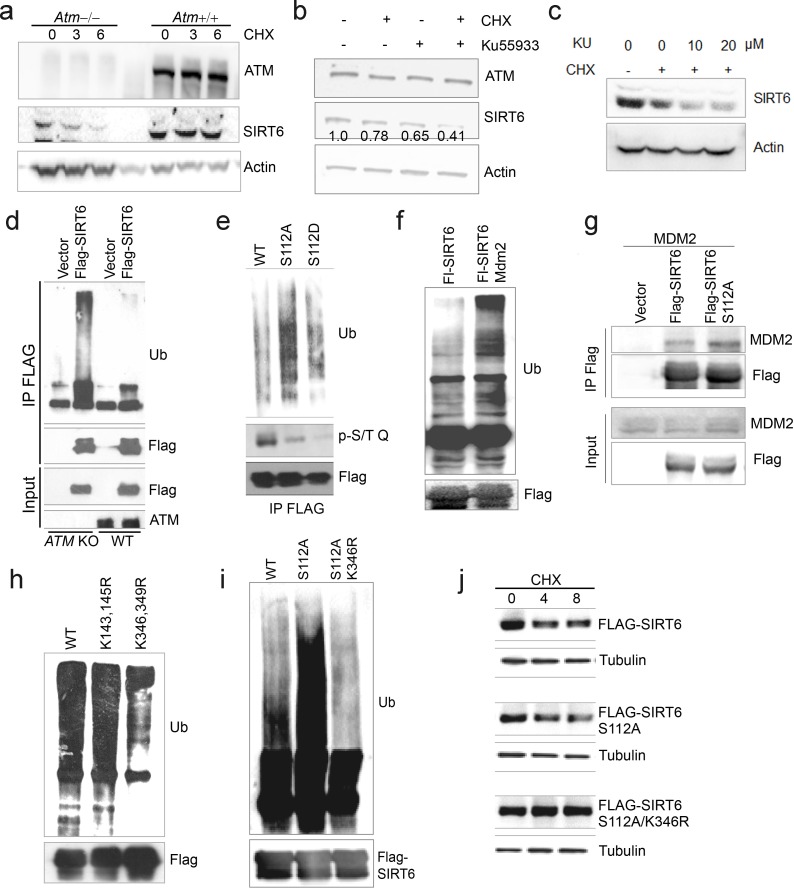
We next examined whether ATM phosphorylates SIRT6. Firstly, we found that CQ or CPT treatment significantly enhanced the binding of SIRT6 to ATM (Figure 3f and Figure 3—figure supplement 1f), whereas the S1981A mutant blocked such association (Figure 3—figure supplement 1g). ATM preferentially phosphorylates the S/T-Q motif. In the presence of CPT, an increased p-S/TQ level of SIRT6 was identified (Figure 3g). Of note, lambda protein phosphatase (λPP) diminished the p-S/TQ level of SIRT6 (Figure 3—figure supplement 1h). Likewise, the p-S/TQ level of SIRT6 was elevated in cells treated with low glucose, which activates ATM by ROS generation (Assaily et al., 2011; Sarre et al., 2012) (Figure 3—figure supplement 1i). Moreover, ectopic ATM significantly increased the p-S/TQ level of SIRT6, but this was abolished in the case of S1981A mutant (Figure 3h). Consistently, a pronounced reduction of p-S/TQ level of SIRT6 was observed in cells lacking ATM or treated with KU55933, a selective and specific ATM kinase inhibitor (Berkovich et al., 2007; Hickson et al., 2004) (Figure 3i–j). The decrease in p-S/TQ level was primarily attributable to loss of ATM, as it was restored by ectopic FLAG-ATM in a dose-dependent manner (Figure 3—figure supplement 1j). Indeed, SIRT6 has one evolutionarily conserved S112Q113 motif (Figure 3k). We therefore constructed S112A and S112D mutants, which resemble hypo- and hyper-phosphorylated SIRT6 respectively. As shown, these mutations almost abolished the pS/T-Q level of FLAG-SIRT6 (Figure 3l). The in vitro kinase assay showed that ATM could phosphorylate GST-SIRT6, but not S112A (Figure 3—figure supplement 1k). Furthermore, compared with SIRT6 S112A, ectopic S112D exhibited a much higher inhibitory effect on glycolytic gene expression in sh-SIRT6 HepG2 cells (Figure 3m and Figure 3—figure supplement 1l), and enhanced chromatin association of SIRT6 (Figure 3n). Collectively, the data suggest that ATM directly phosphorylates SIRT6 at Serine 112.
We next examined whether ATM is involved in regulating SIRT6 protein stability. Notably, compared with WT or vehicle control, the degradation rate of ectopic and endogenous SIRT6 was largely increased in ATM KO HEK293 cells, Atm-/- MEFs, and cells incubated with KU55933 in the presence of cycloheximide (CHX) (Figure 4a–b and Figure 4—figure supplement 1a–c). Recently, MDM2 was demonstrated to ubiquitinate SIRT6 and promote its proteasomal degradation (Thirumurthi et al., 2014). We therefore examined the polyubiquitination level of SIRT6. As shown, the ubiquitination level of FLAG-SIRT6 in ATM KO cells was significantly elevated compared with WT (Figure 4—figure supplement 1d). While S112A mutant markedly enhanced the polyubiquitination level of SIRT6, S112D had little effect (Figure 4—figure supplement 1e). Moreover, S112A accelerated SIRT6 degradation, whereas S112D retarded it (Figure 4c–d), indicating that the Ser112 phosphorylation by ATM regulates SIRT6 ubiquitination and thus protein stability. Indeed, ectopic MDM2 enhanced the polyubiquitination level of FLAG-SIRT6 (Figure 4—figure supplement 1f). In the case of ATM depleted or SIRT6 S112A mutant, the binding capacity of SIRT6 to MDM2 was enhanced (Figure 4e and Figure 4—figure supplement 1g). In searching for key residues that are polyubiquitinated by MDM2, we identified two clusters of lysine residues, i.e. K143/145 and K346/349, which are conserved across species. We then generated KR mutations of these residues, and found that K346/349R remarkably reduced the polyubiquitination level of SIRT6 (Figure 4—figure supplement 1h). Individual KR mutation showed that K346R significantly blocked MDM2-mediated ubiquitination and degradation of SIRT6, whereas K349R hardly affected it (Figure 4f–g). More importantly, K346R restored the increased ubiquitination and accelerated protein degradation of SIRT6 S112A (Figure 4—figure supplement 1i–j). Collectively, these data indicate that K346 is subject to MDM2-mediated ubiquitination, which is inhibited by ATM-mediated S112 phosphorylation.
(a) Immunoblots showing protein levels of Flag-SIRT6 in WT and ATM KO HEK293 cells treated with CHX (50 µg/ml) for indicated periods of time. (b) Quantification of protein levels in (a) by ImageJ. Data represent means ± SEM of three independent experiments. **p<0.01. (c) Immunoblots showing protein levels of Flag-SIRT6, S112A, and S112D in the presence of CHX (50 μg/ml) for indicated periods of time. (d) Quantification of protein levels in (c) by ImageJ. Data represent means ± SEM of three independent experiments. ***p<0.001. (e) Immunoblots showing increased binding capacity between SIRT6 and MDM2 in ATM KO HEK293 cells. (f) Immunoblots showing ubiquitination of Flag-SIRT6, K346R, K349R, and K346/349R (2KR) in HEK293 cells. Note that 2KR and K346R abrogated the ubiquitination of Flag-SIRT6. (g, h) Upper, immunoblots showing protein levels of Flag-SIRT6, K346R, and K349R in the presence of CHX (50 μg/ml) for indicated periods of time. Lower, quantification of protein levels by ImageJ. Data represent means ± SEM of three independent experiments. ***p<0.001.
Figure 4—figure supplement 1.
(a) Immunoblots showing Sirt6 levels in wild-type and Atm null MEFs in the presence of CHX (50 μg/ml). (b–c) Immunoblots showing SIRT6 protein level in HEK293 cells in the presence of CHX (50 μg/ml) and/or KU55933 (10 or 20 μM). Densitometry analysis was performed to determine the SIRT6/actin ratio. (d) Immunoblots showing increased ubiquitination of SIRT6 in ATM KO HEK293 cells. (e) Immunoblots showing ubiquitination of Flag-SIRT6, Flag-SIRT6 S112A, and Flag-SIRT6 S112D in HEK293 cells co-transfected Myc-Ub. (f) Immunoblots showing increased ubiquitination of Flag-SIRT6 in HEK293 cells overexpressing MDM2. (g) Immunoblots showing MDM2 in anti-Flag immunoprecipitates in HEK293 cells expressing Flag-SIRT6 or Flag-SIRT6 S112A. (h) Immunoblots showing ubiquitination of Flag-SIRT6, K143/145R, and K346/349R in HEK293 cells. Noted that K364/349R abrogated the ubiquitination of Flag-SIRT6. (i) Immunoblots showing the ubiquitination of Flag-SIRT6, Flag-SIRT6 S112A, and Flag-SIRT6 S112A/K346R. (j) Immunoblots showing protein levels of Flag-SIRT6, Flag-SIRT6 S112A, and Flag-SIRT6 S112A/K346R in the presence of CHX (50 μg/ml). Note that K346R rescued the accelerated degradation of S112A.
Activating ATM via CQ promotes longevity
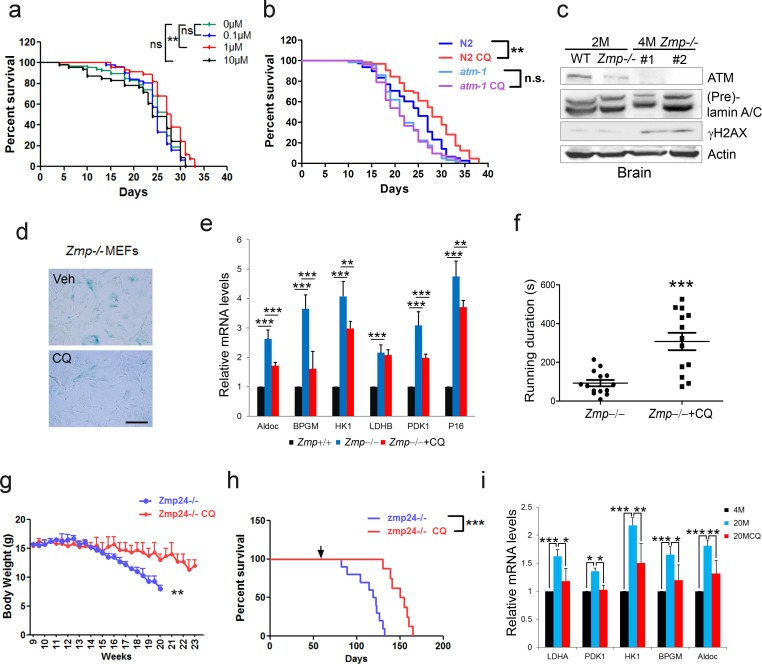
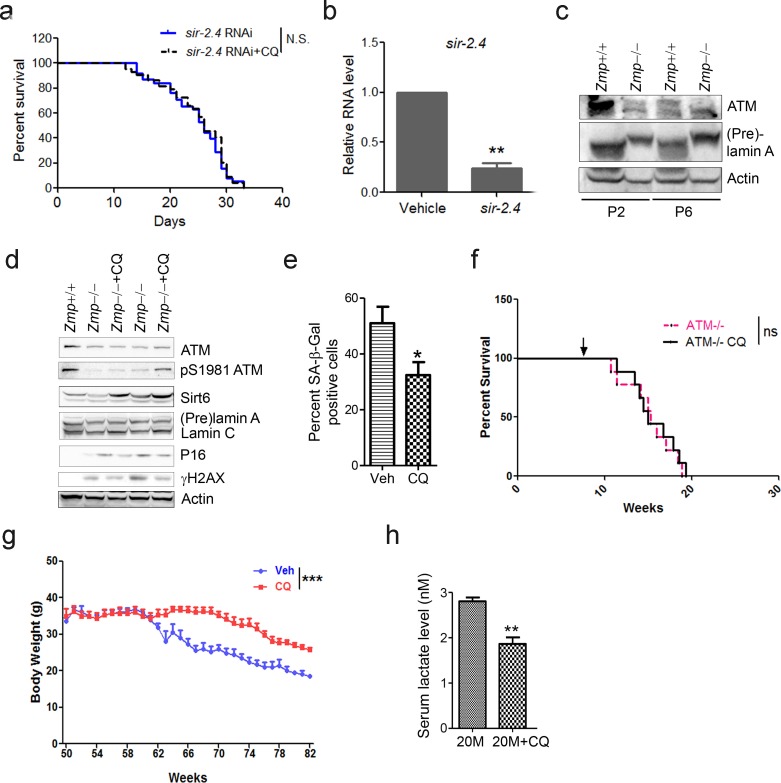
The cellular data suggest a pro-longevity function of ATM. We then tested it at organismal level. We employed Caenorhabditis elegans, which have a short lifespan of approximate 30 days. Nematodes deficient for atm-1, an orthologue of mammalian ATM, and WTs were exposed to various doses of CQ (see Materials and methods). Significantly, the period treatment with CQ (1.0 µM) extended the median lifespan (~14%) of C. elegans (Figure 5a). The lifespan-extending effect was abolished in atm-1 KO (Figure 5b) or in SIRT6 homolog sir-2.4 KD nematodes (Figure 5—figure supplement 1a–b). The data suggest that CQ promotes longevity in an ATM- and SIRT6- dependent manner. We further examined the beneficial effect of CQ in a HGPS model, i.e. Zmpste24-/- mice, which has a shortened lifespan of 4–6 months (Pendás et al., 2002) and impaired ATM-mediated DNA repair signaling (Liu et al., 2013a). We found that the level of Atm was dramatically reduced in Zmpste24-/- MEFs and tissues (Figure 5c and Figure 5—figure supplement 1c). Significantly, CQ treatment activated Atm, stabilized Sirt6, decreased the accumulated DNA damage, inhibited glycolysis, and alleviated senescence in Zmpste24-/- cells (Figure 5d–e and Figure 5—figure supplement 1d–e). The CQ treatment also delayed body weight decline, increased running endurance, and prolonged lifespan in Zmpste24-/- mice (Figure 5f–h), but had no significant effect on the lifespan of Atm-/- mice (Figure 5—figure supplement 1f).
(a) Survival analysis of C. elegans treated with the indicated dosage of CQ. **p<0.01. NS indicates no significant difference. (b) Survival analysis of wild-type and atm-1 null C. elegans cultured in medium with or without 1 µM CQ. (c) Immunoblots showing protein levels of Atm and γH2AX in brain tissues of Zmpste24+/+ (2 months), Zmpste24-/- (2 months), and Zmpste24-/- (4 months) mice. (d) Representative images showing SA-β-Gal staining in Zmpste24-/- MEFs with or without CQ treatment. Scale bar, 100 µm. (e) Quantitative RT-PCR analysis of mRNA levels of p16Ink4a and indicated glycolytic genes in liver tissues of Zmpste24+/+, saline-treated, and CQ-treated Zmpste24-/- mice. Mice were treated for 8 weeks with two weekly intraperitoneal injections of CQ at 3.5 mg/kg. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001. (f) Maximum running duration in saline- and CQ-treated Zmpste24-/- mice. Data represent means ± SEM. ***p<0.001. (g) Body weight of saline- and CQ-treated male Zmpste24-/- mice. Data represent means ± SEM. **p<0.01. (h) Kaplan-Meier survival curves of saline-treated (n = 10) and CQ-treated (n = 8) Zmpste24-/- mice. ***p<0.001. (i) Quantitative RT-PCR analysis of mRNA levels of indicated glycolytic genes in the liver tissues of 4-month-old, saline-treated 12-month-old (n = 3), and CQ-treated 20-month-old (n = 3) mice. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Figure 5—source data 1.
Lifespan analysis of chloroquine's effect on nematodes.Figure 5—source data 2.
Lifespan analysis of chloroquine's effect on mice.Figure 5—figure supplement 1.
(a) Survival analysis of sir-2.4 downregulated C. elegans exposed to medium with or without 1 µM CQ. ‘N.S.’ indicates no significant difference. (b) Quantitative RT-PCR analysis of mRNA levels of sir-2.4 in vehicle and sir-2.4 RNAi-treated C. elegans. (c) Immunoblots showing protein levels of Atm in wild-type and Zmpste24 null MEFs. (d) Immunoblots showing levels of proteins involved in pS1981-ATM, γH2AX, Sirt6, and p16 in liver tissues of saline-treated, and CQ-treated Zmpste24-/- mice. (e) Quantification of SA-β-Gal staining in (Figure 5d) from five views randomly captured for each group. Data represent means ± SEM. *p<0.05. Scale bar, 100 µm. (f) Kaplan-Meier survival curves of saline-treated and CQ-treated Atm-/- mice (n = 9 for each group). ‘ns’ indicates no significant difference. (g) Body weight of saline- and CQ-treated male aging mice. Data represent means ± SEM. ***p<0.001. (h) Serum lactate levels in saline- and CQ-treated 20-month-old mice. Data represent means ± SEM. **p<0.01.
Figure 5—figure supplement 2.
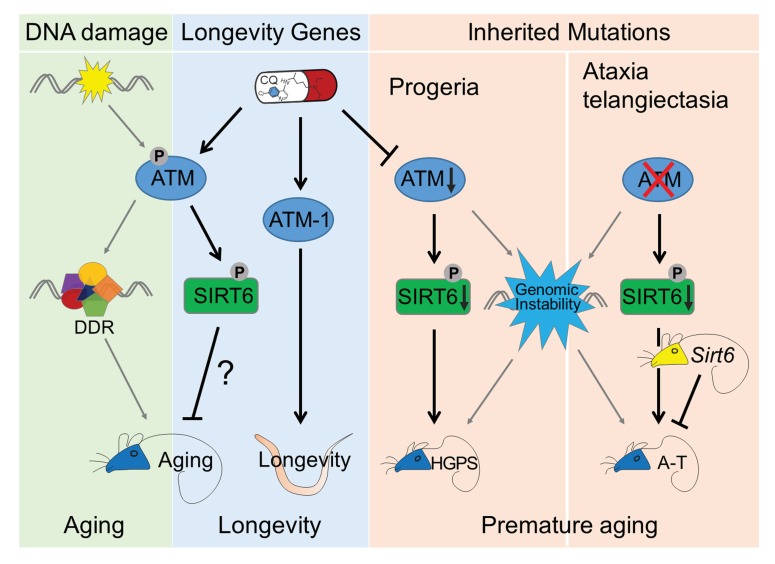
Left, DNA damage activates DDR cascade, and its constant activation leads to permanent cell cycle exit and senescence. Right, defective ATM-SIRT6 axis underlies premature aging in mouse models resembling HGPS and A-T, which is rescued by treatment of CQ and Sirt6 transgene, respectively. Middle, during physiological aging, DNA damage-free activation of ATM by CQ promotes longevity at organismal levels.
Physiologically aged mice frequently develop aging-associated metabolic disorders, with high glucose and lactate (Houtkooper et al., 2011). Given that ATM declines with age, and activation of ATM by CQ inhibits glycolysis in senescent cells and Zmpste24-/- mice, we intraperitoneally administrated 12-month-old ‘old’ male mice with low-dose CQ (3.5 mg/kg) twice a week. Remarkably, compared with the saline-treated group, CQ treatment inhibited glycolysis, lowered serum lactate level, and attenuated body weight decline (Figure 5i and Figure 5—figure supplement 1g–h), implicating potential benefits of CQ in physiologically aged mice. Generally, these data demonstrate a lifespan-extending benefit of ATM activation by CQ.
Discussion
DNA damage accumulates with age, and defective DDR and DNA repair accelerates aging. However, whether boosting DNA repair machinery promotes healthiness and longevity is still unknown. DNA damage stimulates DDR, but if persistent, it instead leads to senescence. Therefore, if enhancing DDR efficacy possibly promotes longevity, it must be DNA damage free. The antimalarial drug CQ can intercalate into the internucleosomal regions of chromatin, unwind DNA helical twist, and thus activate ATM without causing any DNA damage (Bakkenist and Kastan, 2003; Krajewski, 1995). We demonstrate that long-term treatment with CQ activates ATM, improves DNA repair, restores age-related metabolic shift, alleviates cellular senescence, and extends lifespan of nematodes and Zmpste24 null mice. Mechanistically, ATM phosphorylates the longevity gene SIRT6 (Tasselli et al., 2017), and prevents MDM2-mediated ubiquitination and proteasomal degradation of SIRT6. To our knowledge, this is the first study to establish direct causal links between robust DNA repair machinery and longevity. In support of this notion, DNA repair efficacy has been shown to be enhanced in long-lived naked mole rat (MacRae et al., 2015), and human longevity has been shown to be associated with single nucleotide polymorphisms (SNPs) in DNA repair genes/pathways (Debrabant et al., 2014; Soerensen et al., 2012). Interestingly, the heterozygous rather than homozygous status of a SNP, albeit both enhance the transcription of ATM, is associated with longevity in Chinese and Italian populations (Chen et al., 2010; Piaceri et al., 2013). Therefore, in future study, it would be worthwhile evaluating whether Atm can promote longevity in model organisms, and, if so, how many extra copies are required.
Accumulation of DNA damage and metabolic disturbance are common denominators of aging (López-Otín et al., 2013; Moskalev et al., 2013). Metabolic reprogramming from the TCA cycle to glycolysis is prominent in both physiological and pathological aging (Feng et al., 2016; Shimizu et al., 2014). Why senescent cells become glycolytic is poorly understood. The crosstalk between cellular metabolism and DDR is not well elucidated. Upon genotoxic stress, ATM represses the rapamycin-sensitive mammalian target of the rapamycin (mTORC1) pathway (Alexander et al., 2010), but activates the pentose phosphate pathway (PPP) (Cosentino et al., 2011), suggesting that cell metabolism may be key downstream of DDR signaling. Moreover, it is recognized that deficiency in DNA repair machinery such as ATM, WRN, and Ercc1, accelerates aging and causes severe metabolic disorders (Garinis et al., 2008; White and Vijg, 2016). In this study, we showed that boosting ATM activity using a low dose of CQ enhances genomic stability, attenuates age-onset metabolic reprogramming, alleviates senescence, and extends lifespan in mice. The data demonstrate for the first time that enhanced DNA repair machinery (ATM-SIRT6 axis) promotes longevity. Considering that ATM and SIRT6 function not only in maintenance of genome integrity but also as homeostatic protein modifiers, the pro-longevity role of ATM is most likely benefited from enhanced DNA repair and metabolic homeostasis, but it is difficult to determine which is more important.
Recently, Bohr’s group identified that increased consumption of NAD+ by an early DDR factor poly (ADP-ribose) polymerase (PARP1), owing to accumulated DNA damage, accelerated aging in Atm mutant mice (Fang et al., 2016). NAD+ serves as a cofactor of sirtuins, including SIRT1 and SIRT6. Therefore, this work establishes a linear causal link between deficient DDR, DNA damage accumulation, consumption of NAD+, decline in sirtuin activity, and aging. Moreover, administration of nicotinamide mononucleotide or nicotinamide riboside ameliorates age-related function decline and extends lifespan in mice (Mills et al., 2016; Zhang et al., 2016). Here, we found that ATM decline during aging causes DNA damage accumulation and enhances glycolysis, both of which consume most of the NAD+, providing an explanation for the low NAD+ level in Atm-/- mice and physiologically aged mice.
Closely resembling normal aging, HGPS has attracted numerous efforts in understanding of molecular mechanisms and developing therapeutic strategies (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003). We and others have found that HGPS and Zmpste24 null cells undergo premature senescence because of defective chromatin remodeling (Ghosh et al., 2015; Krishnan et al., 2011; Liu et al., 2013a; Liu et al., 2013b), delayed DDR, and impaired DNA repair (Liu et al., 2005; Liu et al., 2013b; Varela et al., 2005). Specifically, Atm-Kap-1 signaling is compromised (Liu et al., 2013a), and SIRT6 protein level and deacetylase activity are reduced in progeria cells (Endisha et al., 2015; Ghosh et al., 2015). Here we showed that Atm is significantly downregulated, which explains the reduced SIRT6, delayed DDR, and metabolic shift in progeria cells and mice. It would be interesting to investigate whether ectopic Atm or Sirt6 could rescue progeroid features in these mice. Nevertheless, the activation of ATM via CQ remarkably improves glucose homeostasis, DNA damage clearance, and running endurance, and extends lifespan in progeria mice. It would be worthwhile evaluating the pro-longevity benefits of CQ in physiological aging.
CQ is an FDA-approved and clinically used medicine for treatment of malaria (2015). Via activation of ATM, long-term treatment of CQ protects against atherosclerosis, improves insulin sensitivity, and rescues glucose tolerance in type 2 diabetes (T2D) (Emami et al., 1999; Razani et al., 2010; Schneider et al., 2006). The lysosomotropic property of CQ also makes it a potent inhibitor of autophagy (Yang et al., 2013). The application of CQ for antimalarial treatment (500 mg/week, maximum 0.8 µM in plasma) and for cancer therapy (100–500 mg/day) (Kimura et al., 2013) is attributed to its inhibitory action on autophagy. Of note, CQ also the attenuates inflammatory response by inhibiting autophagy (Szatmári-Tóth et al., 2016; Whelan et al., 2017; Wu et al., 2018), requiring a high dosage of 50 mg/kg for mice. In the current study, we used a low dose of CQ to activate ATM, i.e. 1–10 μM for cell line and 3.5 mg/kg twice a week for mice (Schneider et al., 2006). The results showed that at such low doses, CQ has no toxicity and little effect on basal autophagic activity. Moreover, a low dose of CQ prolongs lifespan in progeroid mice, but exhibits little effect on Atm KO background, supporting an ATM-dependent pro-longevity function of CQ. Unfortunately, we could not test the CQ effect in Sirt6-/- mice with only 1-month lifespan (Mostoslavsky et al., 2006b). Here, we addressed the pro-longevity benefits of CQ-activated ATM, most likely attributable to improved DNA repair and glucose metabolism. Given that ATM also displays anti-inflammatory function (Erttmann et al., 2017; Shoelson, 2006), we could not rule out an anti-inflammatory effect in lifespan extension observed in CQ-treated mice.
In conclusion, our data establish direct causal links between robust DNA repair machinery and longevity. In line with DNA damage theory of aging, we propose that DNA damage activates DDR; however, its constant activation causes senescence; defective ATM-SIRT6 axis underlies premature aging, exemplified by HGPS and A-T mouse models, which are rescued by treatment of CQ and Sirt6 transgene, respectively; in physiological aging, DNA damage-free activation of ATM by CQ stabilizes SIRT6, thus promoting longevity in nematodes and most likely also in mice (Figure 5—figure supplement 2). Our findings provide a novel therapeutic strategy for HGPS, and could facilitate clinical trials of CQ as an effective treatment for age-related diseases.
Materials and methods
Key resources table
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (human) | ATM | National Center for Biotechnology Information | Gene ID: 472 | |
| Gene (mouse) | Atm | National Center for Biotechnology Information | Gene ID: 11920 | |
| Gene (Caenorhabditis elegans) | atm-1 | National Center for Biotechnology Information | Gene ID: 3565793 | |
| Gene (human) | SIRT6 | National Center for Biotechnology Information | Gene ID: 51548 | |
| Gene (mouse) | Sirt6 | National Center for Biotechnology Information | Gene ID: 50721 | |
| Gene (Caenorhabditis elegans) | sir-2.4 | National Center for Biotechnology Information | Gene ID: 182284 | |
| Gene (mouse) | Zmpste24 | National Center for Biotechnology Information | Gene ID: 230709 | |
| Gene (mouse) | p53 | National Center for Biotechnology Information | Gene ID: 230710 | |
| Cell line (human) | HEK293 | ATCC | Catalog number: ATCC CRL-1573; RRID:CVCL_0042 | |
| Cell line (human) | HepG2 | ATCC | Catalog number: ATCC HB-8065; RRID:CVCL_0027 | |
| Cell line (human) | U2OS | ATCC | Catalog number: ATCC HTB-96; RRID:CVCL_0042 | |
| Cell line (mouse) | Atm-/-; p53-/- MEF | from Dr. Yosef Shiloh (Tel Aviv University, Israel) | ||
| Cell line (mouse) | Sirt6-/- MEF | from Dr. Raul Mostoslavsky (Massachusetts General Hospital Cancer center, USA) | ||
| Antibody | ATM | Abcam (Cambridge, UK) | Cat# ab78; RRID:AB_306089 | Applications: WB;Immunofluorescence |
| Antibody | SIRT6 | Abcam (Cambridge, UK) | Cat# ab62739; RRID:AB_956300 | Applications: WB; Immunofluoresce;Chromatin immunoprecipitation |
| Antibody | γH2AX | Abcam (Cambridge, UK) | Cat# ab81299; RRID:AB_1640564 | Applications: WB |
| Antibody | p21 | Santa Cruz Biotechnology | Cat# sc-6246; RRID:AB_628073 | Applications: WB |
| Antibody | MDM2 | Santa Cruz Biotechnology | Cat# sc-965; RRID:AB_627920 | Applications: WB |
| Antibody | p-ATM (Ser1981) | EMD Millipore | Cat# 05–740; RRID:AB_309954 | Applications: WB |
| Antibody | H3K9ac | EMD Millipore | Cat# 07–352; RRID:AB_310544 | Applications: WB; Chromatin immunoprecipitation |
| Antibody | p-S/T Q | Cell Signaling Technology (Beverly, MA) | Cat #9607S; RRID:AB_10889739 | Applications: WB |
| Antibody | cleaved caspase-3 | Cell Signaling Technology (Beverly, MA) | Cat #9661; RRID:AB_2341188 | Applications: WB |
| Antibody | HA | Sigma-Aldrich | Cat# H3663; RRID:AB_262051 | Applications: WB |
| Antibody | Flag | Sigma-Aldrich | Cat# F1804; RRID: AB_262044 | Applications: WB |
| Antibody | LC3B | Sigma-Aldrich | Cat# L7543; RRID:AB_796155 | Applications: WB |
| Transfected construct (human) | Flag-His-ATM wt | Addgene (Cambridge, MA) | Cat #31985 | |
| Transfected construct (human) | Flag-SIRT6 | Addgene (Cambridge, MA) | Cat #13817 | |
| Transfected construct (human) | Flag-His-ATM S1981A | Addgene (Cambridge, MA) | Cat #32300 | |
| Commercial assay or kit | Senescence beta- galactosidase staining Kit | Cell Signaling Technology (Beverly, MA) | Cat #9860 | |
| Commercial assay or kit | Lactate Colorimetric Assay Kit | BioVision | Cat #K667-100 | |
| Commercial assay or kit | Click-iT EdU Alexa Fluor 488 Kit | Invitrogen | Cat #{"type":"entrez-nucleotide","attrs":{"text":"C10425","term_id":"1535496","term_text":"C10425"}}C10425 | |
| Chemical compound, drug | Cycloheximide | Sigma-Aldrich | Cat #66-81-9 | |
| Chemical compound, drug | MG-132 | Sigma-Aldrich | Cat #474787 | |
| Chemical compound, drug | Chloroquine | Sigma-Aldrich | Cat #C6628 |
Mice
Zmpste24-/- mice and Atm-/- mice have been described previously (Barlow et al., 1996; Pendás et al., 2002). Sirt6-transgenic mice (Sirt6-tg) of C57BL/6J background were constructed by injecting cloned mSirt6 cDNA with CAG promoter into fertilized eggs. Primers for genotyping of Sirt6 transgenic allele were as follows: forward: 5’-CTGGTTATTGTGCTGTCTCATCAT-3’; reverse: 5’-CCGTCTACGTTCTGGCTGAC-3’. Atm-/- mice were crossed to Sirt6-tg mice to get Atm-/-;Sirt6-tg mice. Chloroquine (CQ) experiments were conducted as described (Schneider et al., 2006). Briefly, 12-month-old wild-type C57BL/6J male mice, 2-month-old Zmpste24-/-, and Atm-/- male mice were administered with CQ (Sigma, St. Louis, MO) in 0.9% saline twice per week at 7 mg/kg body weight, and the control group was treated with saline alone. At least 8 weeks after treatment of CQ, mice were subjected to functional tests. Body weight and lifespan was recorded. The survival rate was analyzed using the Kaplan–Meier method and statistical comparison was performed using the Log-rank Test. Mice were housed and handled in the laboratory animal research center of Shenzhen University. All experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). The protocols were approved by the Animal Welfare and Research Ethics Committee of Shenzhen University (Approval ID: 201412023).
C. elegans survival assay
C. elegans nematode survival assay was performed according to standard protocols (Kenyon et al., 1993). Briefly, wild-type and atm-1 null nematodes (100 to 150 per group) synchronized to prefertile young adult stage were exposed to NGM plates containing the indicated dosage of CQ. After 1-day incubation, animals were transferred to fresh incubation plates without CQ for another 2 days. This procedure was repeated every 3 days. Nematodes that showed no response to gentle stimulation were recorded as dead. The survival data were analyzed using the Kaplan–Meier method and statistical comparison was performed using the Log-rank Test.
Cell lines
HEK293 (CRL-1573), HepG2 (HB-8065), and U2OS (HTB-96) cells were purchased from ATCC. Human skin fibroblasts HSFs (F2-S) and primary MEFs were prepared as described previously (Liu et al., 2005). Immortalized Atm-/-; p53-/- and Sirt6-/- MEFs were provided as a kind gift from Dr. Yosef Shiloh (Tel Aviv University, Israel) and Dr. Raul Mostoslavsky (Massachusetts General Hospital Cancer center, USA), respectively. These cell lines were authenticated by short tandem repeat (STR) profile analysis and genotyping, and were mycoplasma free. Cells were cultured in Gibco DMEM (Life Technologies, USA) with 10% fetal bovine serum (FBS), 100 U/ml penicillin and streptomycin (P/S) at 37°C in 5% CO2 and atmospheric oxygen conditions. For CQ experiments, cells were maintained in the medium containing 1 μM chloroquine for 12 hr, and then grown in new fresh medium for 48 hr.
Plasmids
Human Flag-SIRT6, pcDNA3.1 Flag-ATM, Flag-ATM S1981A, and pcDNA3 human MDM2 were all purchased from Addgene (Cambridge, MA). Flag-SIRT6 with amino acid substitution mutations (S112A, S112D, K346R/K349R) were generated by PCR-based mutagenesis using pcDNA3-Flag-SIRT6 as a template and a QuikChange II site-directed mutagenesis kit (Agilent Technologies), following the manufacturer’s instructions. Primer sequences for amino acid mutations of SIRT6 were as follows: SIRT6 S112A: (forward) 5'-cgtccacgttctgggcgaccaggaagcgga-3', (reverse) 5’-tccgcttcctggtcgcccagaacgtggacg-3’; SIRT6 S112D: (forward) 5'-ccgtccacgttctggtcgaccaggaagcggag-3', (reverse) 5'-ctccgcttcctggtcgaccagaacgtggacgg-3'; SIRT6 K346R: (forward) 5'-ggccttcacccttctggggggtctgtg-3', (reverse) 5'-cacagaccccccagaagggtgaaggcc-3'; SIRT6 K349R: (forward) 5'-gccttggccctcacccttttggggggt-3', (reverse) 5'-accccccaaaagggtgagggccaaggc-3. HA-tagged human SIRT6 plasmid was amplified from the respective cDNAs and constructed into pKH3-HA vector. To express four truncated forms of SIRT6 protein, HA-SIRT6 plasmid as a template was constructed by PCR-based deletion.
Protein extraction and western blotting
For whole cell protein extraction, cells were suspended in five volumes of suspension buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, protease inhibitor cocktail), and then five volumes of 2X SDS loading buffer were added and incubated at 98°C for 6 min. Mice tissues were homogenized with 1 ml of ice-cold tissue lysis buffer (25 mM TrisHCl, pH 7.5, 10 mM Na3VO4, 100 mM NaF, 50 mM Na4P2O7, 5 mM EGTA, 5 mM EDTA, 0.5% SDS, 1% NP-40, protease inhibitor cocktail). After homogenization and sonication, lysates were centrifuged at 16,000 g for 15 min. The clean supernatant was carefully transferred to new tubes. Protein concentrations were determined using a bicinchoninic acid (BCA) assay method (Pierce, Rockford, IL) and were normalized with lysis buffer for each sample. Samples were denatured in 1X SDS loading buffer by boiling at 98°C for 6 min. Proteins were separated by loading to SDS-polyacrylamide gels, and then were transferred to PVDF membrane (Millipore). The protein levels were determined by immunoblotting using respective antibodies. The ImageJ program was used for densitometric analysis of immunoblotting, and the quantification results were normalized to the loading control.
Antibodies
Rabbit anti-SIRT6 (ab62739), ATM (ab78), SIRT1 (ab12193), γH2AX (ab81299), RAP80 (ab52893), Kap-1 (ab10484), and p-KAP-1 (Ser824, ab70369) antibodies were obtained from Abcam (Cambridge, UK). Anti-lamin A/C (sc-20681), p21 (sc-6246), MDM2 (sc-965), and P53 (sc-6243) antibodies were purchased from Santa Cruz Biotechnology. Rabbit anti-γH2AX (05–636), p-ATM (Ser1981) (05–740), histone H3 (07–690), anti-H3K56ac (07–677), and H3K9ac (07–352) antibodies were sourced from EMD Millipore. Mouse anti-p-ATM (Ser1981) (#5883), p-S/TQ (#9607), ubiquitin (#3936), and cleaved caspase-3 (#9661) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti- HA, Flag, rabbit anti-LC3B, and P62 antibodies were obtained from Sigma-Aldrich. Anti-Nbs1 (NB100-143) antibody was purchased from Novus Biologicals. Mouse anti-actin, tubulin antibodies were obtained from Beyotime. Anti-pS112 SIRT6 monoclonal antibodies were prepared by Abmart generated from a specific phosphorylated peptide (peptide sequence CLRFVSPQNV).
Protein degradation assay
HEK293 cells (WT and ATM-deficient cells) were transfected with Flag-SIRT6 alone or together with Mdm2. 48 hr later, the cells were treated with 50 μg/ml of cycloheximide (CHX, Sigma-Aldrich), a translation inhibitor. For endogenous SIRT6 protein degradation assay, ATM wild-type and null MEFs were grown in 6 cm plates, and were treated with 50 mg/ml CHX for indicated time points. Cells were collected and the protein levels were determined by western blotting, the subsequent quantification was performed with ImageJ software.
In vivo ubiquitination assay
In vivo ubiquitination assay was performed by transfecting HEK293 cells in 6 cm dishes with 1 μg Myc-ubiquitin, 2 μg Flag-SIRT6 or its mutations, and/or 1 μg MDM2 vector. 48 hr after transfection, cells were lysed in the buffer (25 mM Tris-HCl pH 8.0, 250 mM NaCl, 10 mM Na3VO4, 1 mM EDTA, 10% glycerol, protease inhibitor cocktail, and 0.1 mM phenylmethylsulphonyl fluoride), and then incubated with Flag-M2 beads (Sigma-Aldrich) overnight at 4°C. Beads were washed with lysis buffer three times, bound proteins were eluted by adding 1.5 × SDS loading buffer. The ubiquitin levels were analyzed by immunoblotting.
In vitro kinase assay
HEK293T cells were transfected with 10 μg of FLAG-ATM and then treated with CPT. Activated ATM was immune-purified from the cell extracts with FLAG beads (Sigma, M8823). GST-SIRT6 or the S112A mutant was purified from bacteria. Kinase reactions were initiated by incubating purified ATM with GST-SIRT6 in the kinase buffer with or without 1 mM ATP for 120 min at 30°C. After reaction, proteins were blocked by SDS loading buffer. The membrane was then subjected to western blotting with antibodies against p-S/TQ.
Immunoprecipitation
Cells under indicated treatments were totally lysed in lysis buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 10 mM Na3VO4, 10% glycerol, 2 mM EDTA, protease inhibitor cocktail, and 0.1 mM phenylmethylsulphonyl fluoride. After sonication and centrifugation, the supernatant was collected and incubated with H3K9ac (Millipore, 2 μg/sample) overnight at 4°C with a gentle rotation. Protein A/G agarose (Pierce, 10 μl/sample) were added to the tubes and rotated at 4°C for 2 hr. Beads were precipitated by centrifugation at 1000 g for 15 s and washed three times with cold lysis buffer. The pellet was resuspended in 1.5 × SDS loading buffer and incubated at 98°C for 6 min. The supernatants were collected and used for western blotting.
GST pull-down assay
A series of GST fusion proteins of truncated ATM, which together spanned the full length of ATM, were constructed into pGEX4T-3 vector. For GST pull-down, bacterially expressed 6 × His tagged SIRT6 was separately incubated with various GST-ATM fragments in a buffer of 150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 0.2 mM EDTA, 10% glycerol, 0.2% NP-40, and protease inhibitors (Roche Complete). GST-fusion proteins were then precipitated by adding Glutathione Sepharose fast flow (GE Healthcare). After washing twice with TEN buffer (0.5% Nonidet P-40, 20 mM Tris-HCl [pH 7.4], 0.1 mM EDTA, and 300 mM NaCl), glutathione agarose beads were analyzed by western blotting and coomassie staining.
RNA interference and shRNA lentiviral infection
Briefly, cells were transfected with small interfering RNAs (siRNAs) for 48 hr using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s instructions. The siRNAs targeting human ATM, SIRT6, and HDM2 were purchased (GenePharma, China) with sequences as follows, si-ATM#1: 5’-AAUGUCUUUGAGUAGUAUGUU-3’ (Zhou et al., 2003); Si-ATM#2: 5’-AAGCACCAGUCCAGUAUUGGC-3’ (Zhang et al., 2005); si-SIRT6#1: 5’-AAGAAUGUGCCAAGUGUAAGA-3’; si-SIRT6#2: 5’-CCGGCTCTGCACCGTGGCTAA-3’; si-HDM2#1: 5’-AACGCCACAAATCTGATAGTA-3’; si-HDM2#2: 5’-AATGCCTCAATTCACATAGAT-3’. A scrambled siRNA sequence was used as control. Lentiviral shRNA constructs were generated in a pGLVH1 backbone (GenePharma, China), and virus was produced in HEK293 cells. To deplete ATM in HSF cells and SIRT6 in HepG2 cells, lentiviral infection was performed in the presence of 5 μg/ml polybrene. Two days later, the infected HSF cells or HepG2 cells were selected with 2 μg/ml puromycin. To downregulate sir-2.4 expression, the NL2099 worms were exposed to incubation plates containing HT115 bacteria with sir-2.4 RNAi vector.
CRISPR/Cas9-mediated genome editing
Gene mutagenesis by the CRISPR/Cas9 system was conducted as described (Ran et al., 2013). The following gRNAs targeting human ATM, SIRT6 were constructed in pX459 vector (Addgene, #48139). sgATM F: 5’-CACCGATATGTGTTACGATGCCTTA-3’, R: 5’- AAACTAAGGCATCGTAACACATATC-3’. HEK293 cells were transfected with pX459 or pX459-gRNA using Lipofetamine 3000 Transfection Reagent according to the manufacturer's instructions. After 2-day culture, cells were selected with 2 μg/ml puromycin, six colonies were picked and grown to establish stable cell lines. The targeted mutations were identified by western blotting, and PCR-based sequencing.
EdU (5-ethynyl-2’-deoxyuridine) incorporation assay
EdU incorporation assays were conducted in HSF cells to estimate cell proliferation using the Click-iT EdU Alexa Fluor 488 Kit (Invitrogen, USA). HSF cells, infected by the respective lentiviruses containing shNC and shATM, were cultured in a six-well plate containing the coverslips in the presence of 10 μM EdU for 12 hr. Cells were fixed in 3.7% formaldehyde followed by 0.5% Triton X-100 permeabilization, and then stained with Alexa Fluor picolyl azide. Five random views were captured to calculate the positive staining rate for each group.
Growth curves and SA-β-gal assays
Cell population doublings were monitored using a Coulter Counter. SA-β-galactosidase assay in primary cells was performed using Senescence β-galactosidase staining Kit (#9860, CST) according to the manufacturer's instructions. Five views were captured randomly to calculate the positive staining rate for each group.
RNA preparation and Real-Time qPCR
Total RNA was extracted from cells or mouse tissues using Trizol reagent RNAiso Plus (TaKaRa, Japan) following the phenol–chloroform extraction method. Purified total RNA was used to obtain cDNA using PrimeScript RT Master Mix (Takara, Japan) following this method: 37°C for 30 min, and 85°C for 5 s. The gene expression was analyzed with the CFX Connected Real-Time PCR Detection System (BioRad) with SYBR Ex Taq Premixes (Takara, Japan). Gene expression levels were normalized to actin.
Glucose tolerance test
Mice were fasted overnight (6 p.m. to 9 a.m.), and D-glucose (2.5 g/kg body weight) was administrated intraperitoneally. Blood glucose levels were determined from tail vein blood using a glucometer (Onetouch ultravue, Johnson, USA) at 0, 30, 60, 90, and 120 min after D-glucose injection.
Insulin tolerance test
Mice were fasted for 6 hr (8 a.m. to 2 p.m.), and recombinant human insulin (0.75 U/kg body weight) was administered intraperitoneally. Blood glucose levels were determined in tail vein blood using a glucometer (Onetouch ultravue, Johnson) at 0, 30, 60, 90, and 120 min after insulin injection.
Lactate assay
Mouse serum was five-fold diluted, and lactate concentration was determined with the Lactate Colorimetric Assay Kit (BioVision).
Endurance running test
Zmpste24-/- mice were treated for 8 weeks with chloroquine or saline before running on a Rota-Rod Treadmill (YLS-4C, Jinan Yiyan Scientific Research Company, Shandong, China) to test the effect of chloroquine on fatigue resistance. Mice were placed on the rotating lane, and the speed was gradually increased to 10 r/min. When mice were exhausted and safely dropped from the rotating lane, the time latency to fall was automatically recorded.
Metabolite analysis
Wild-type and ATM KO cells were grown in normal medium for 24 hr, and methanol-fixed cell pellets were analyzed by a two liquid chromatography-tandem mass spectrometry (LC-MS) method as described (Luo et al., 2007).
Immunofluorescence microscopy
The cells were fixed using 4% paraformaldehyde at room temperature for 15 min, permeabilized by 0.5% Triton X-100 at room temperature for 10 min, blocked using 10% FBS/PBS, and then incubated with primary antibodies diluted in PBS containing 2% BSA overnight at 4°C. The primary antibodies were detected using an Alexa-488-conjugated anti-mouse secondary antibody (Invitrogen). The nuclei were stained using DAPI in anti-fade mounting medium. Images were captured using a Zeiss LSM880 confocal/multiphoton microscope.
ChIP assay
Cells were fixed in 1% formaldehyde for 10 min at room temperature. The crosslinking reaction was quenched with 0.125 M glycine. After washing with PBS, cells were lysed with lysis buffer (50 mM Tris·HCl pH 8.0, 2 mM EDTA, 15 mM NaCl, 1% SDS, 0.5% deoxycholate, protease inhibitor cocktail, 1 mM PMSF), followed by sonication and centrifugation. The supernatant was collected and precleared in dilution buffer (50 mM Tris-HCl pH 8.0, 2 mM EDTA, 150 mM NaCl, 1% Triton X-100) with protein A/G Sepharose and pre-treated salmon DNA. The precleared samples were incubated overnight with H3K9ac antibody (2 μg/sample, Millipore) or appropriate control IgGs (Santa Cruz), and protein A/G Sepharose (Invitrogen). After washing sequentially with a series of buffers, the beads were heated at 65°C to reverse the crosslink. DNA fragments were purified and analyzed. Real-time PCR was performed with primers as described (Zhong et al., 2010):
LDHB-ChIP-5’: AGAGAGAGCGCTTCGCATAG
LDHB-ChIP-3’: GGCTGGATGAGACAAAGAGC
ALDOC-ChIP-5’: AAGTGGGGCACTGTTAGGTG
ALDOC-ChIP-3’: GTTGGGGATTAAGCCTGGTT
PFKM-ChIP-5’: TTAAGACAAAGCCTGGCACA
PFKM-ChIP-3’: CAACCACAGCAATTGACCAC
LDHA-ChIP-5’: AGGGGGTGTGTGAAAACAAG
LDHA-ChIP-3’: ATGGCTTGCCAGCTTACATC
LDHA-ChIP-1Kb-5’: TGCAAGACAAGTGTCCCTGT
LDHA-ChIP-1Kb-3’: GAGGGAATGAAGCTCACAGC
Statistical analysis
Statistical analyses were conducted using two-tailed Student's t-test between two groups. All data are presented as mean ± S.D. or mean ± S.E.M. as indicated, and a p value < 0.05 was considered statistically significant.
Acknowledgements
We thank Dr. Yosef Shiloh (Tel Aviv University, Israel) for Atm-/-; p53-/- MEF cells and Dr. Raul Mostoslavsky (Massachusetts General Hospital Cancer center, USA) for Sirt6-/- MEFs. This project is supported by research grants from the National Natural Science Foundation of China (81422016, 91439133, 81571374, 81501206, 81501210), National Key R and D Program of China (2017YFA0503900, 2016YFC0904600), Research Grant Council of Hong Kong (773313, HKU2/CRF/13G), Natural Science Foundation of Guangdong Province (2014A030308011, 2015A030308007, 2016A030310064) and Shenzhen Science and Technology Innovation Commission (CXZZ20140903103747568, JCYJ20160226191451487 and JCYJ20140418095735645) and Discipline Construction Funding of Shenzhen (2016).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
- Natural Science Foundation of Guangdong Province 2016A030310064 to Minxian Qian.
- National Natural Science Foundation of China 81501206 to Minxian Qian.
- National Natural Science Foundation of China 81501210 to Mingyan Zhou.
- Natural Science Foundation of Guangdong Province 2015A030308007 to Baoming Qin.
- Shenzhen Science and Technology Innovation Commission JCYJ20140418095735645 to Zimei Wang.
- Research Grant Council of Hong Kong HKU2/CRF/13G to Zhongjun Zhou.
- National Natural Science Foundation of China 81422016 to Baohua Liu.
- Ministry of Science and Technology of the People's Republic of China 2017YFA0503900 to Baohua Liu.
- Natural Science Foundation of Guangdong Province 2014A030308011 to Baohua Liu.
- Shenzhen Science and Technology Innovation Commission CXZZ20140903103747568 to Baohua Liu.
- National Natural Science Foundation of China 91439133 to Baohua Liu.
- National Natural Science Foundation of China 81571374 to Baohua Liu.
- Ministry of Science and Technology of the People's Republic of China 2016YFC0904600 to Baohua Liu.
- Shenzhen Science and Technology Innovation Commission JCYJ20160226191451487 to Baohua Liu.
- Research Grant Council of Hong Kong 773313 to Baohua Liu.
- Discipline Construction Funding of Shenzhen to Baohua Liu.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Investigation, Writing—review and editing.
Formal analysis, Investigation, Methodology.
Validation, Investigation.
Methodology.
Resources.
Resources.
Resources.
Resources.
Project administration.
Methodology.
Resources, Methodology, Writing—review and editing.
Resources, Methodology, Writing—review and editing.
Methodology.
Methodology, Writing—review and editing.
Resources, Methodology, Writing—review and editing.
Conceptualization, Resources, Formal analysis, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Ethics
Animal experimentation: Mice were housed and handled in the laboratory animal research center of Shenzhen University. All experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). The protocols were approved by the Animal Welfare and Research Ethics Committee of Shenzhen University (Approval ID: 201412023).
Additional files
Transparent reporting form
Data availability
Sequencing data have been deposited in GEO under accession code {"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280.
The following dataset was generated:
Qian MLiu B2018Boosting ATM activity alleviates ageing and extends lifespan in a mouse model of progeria.https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc={"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280Publicly available at the NCBI Gene Expression Omnibus (accession no: {"type":"entrez-geo","attrs":{"text":"GSE109280","term_id":"109280"}}GSE109280).
Reference
- Aird KM, Worth AJ, Snyder NW, Lee JV, Sivanand S, Liu Q, Blair IA, Wellen KE, Zhang R. ATM couples replication stress and metabolic reprogramming during cellular senescence. Cell Reports. 2015;11:893–901. doi: 10.1016/j.celrep.2015.04.014. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Alexander A, Kim J, Walker CL. ATM engages the TSC2/mTORC1 signaling node to regulate autophagy. Autophagy. 2010;6:672–673. doi: 10.4161/auto.6.5.12509. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Assaily W, Rubinger DA, Wheaton K, Lin Y, Ma W, Xuan W, Brown-Endres L, Tsuchihara K, Mak TW, Benchimol S. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Molecular Cell. 2011;44:491–501. doi: 10.1016/j.molcel.2011.08.038. [PubMed] [CrossRef] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [PubMed] [CrossRef] [Google Scholar]
- Balajee AS, Machwe A, May A, Gray MD, Oshima J, Martin GM, Nehlin JO, Brosh R, Orren DK, Bohr VA. The Werner syndrome protein is involved in RNA polymerase II transcription. Molecular Biology of the Cell. 1999;10:2655–2668. doi: 10.1091/mbc.10.8.2655. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Bar RS, Levis WR, Rechler MM, Harrison LC, Siebert C, Podskalny J, Roth J, Muggeo M. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. The New England Journal of Medicine. 1978;298:1164–1171. doi: 10.1056/NEJM197805252982103. [PubMed] [CrossRef] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/S0092-8674(00)80086-0. [PubMed] [CrossRef] [Google Scholar]
- Berkovich E, Monnat RJ, Kastan MB. Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nature Cell Biology. 2007;9:683–690. doi: 10.1038/ncb1599. [PubMed] [CrossRef] [Google Scholar]
- Boder E, Sedgwick RP. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958;21:526–554. [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. Journal of Biological Chemistry. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [PubMed] [CrossRef] [Google Scholar]
- Chen T, Dong B, Lu Z, Tian B, Zhang J, Zhou J, Wu H, Zhang Y, Wu J, Lin P, Zhang J, Xu H, Mo X. A functional single nucleotide polymorphism in promoter of ATM is associated with longevity. Mechanisms of Ageing and Development. 2010;131:636–640. doi: 10.1016/j.mad.2010.08.009. [PubMed] [CrossRef] [Google Scholar]
- Cooper MP, Machwe A, Orren DK, Brosh RM, Ramsden D, Bohr VA. Ku complex interacts with and stimulates the Werner protein. Genes & Development. 2000;14:907–912. [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. The EMBO Journal. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Lévy N. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [PubMed] [CrossRef] [Google Scholar]
- Debrabant B, Soerensen M, Flachsbart F, Dato S, Mengel-From J, Stevnsner T, Bohr VA, Kruse TA, Schreiber S, Nebel A, Christensen K, Tan Q, Christiansen L. Human longevity and variation in DNA damage response and repair: study of the contribution of sub-processes using competitive gene-set analysis. European Journal of Human Genetics. 2014;22:1131–1136. doi: 10.1038/ejhg.2013.299. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Emami J, Gerstein HC, Pasutto FM, Jamali F. Insulin-sparing effect of hydroxychloroquine in diabetic rats is concentration dependent. Canadian Journal of Physiology and Pharmacology. 1999;77:118–123. doi: 10.1139/y98-146. [PubMed] [CrossRef] [Google Scholar]
- Endisha H, Merrill-Schools J, Zhao M, Bristol M, Wang X, Kubben N, Elmore LW. Restoring SIRT6 expression in hutchinson-gilford progeria syndrome cells impedes premature senescence and formation of dysmorphic nuclei. Pathobiology. 2015;82:9–20. doi: 10.1159/000368856. [PubMed] [CrossRef] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [PubMed] [CrossRef] [Google Scholar]
- Erttmann SF, Härtlova A, Sloniecka M, Raffi FAM, Hosseinzadeh A, Edgren T, Rofougaran R, Resch U, Fällman M, Ek T, Gekara NO. Loss of the DNA damage repair kinase atm impairs inflammasome-dependent anti-bacterial innate immunity. Immunity. 2017;47:1210–1211. doi: 10.1016/j.immuni.2017.11.014. [PubMed] [CrossRef] [Google Scholar]
- Espach Y, Lochner A, Strijdom H, Huisamen B. ATM protein kinase signaling, type 2 diabetes and cardiovascular disease. Cardiovascular Drugs and Therapy. 2015;29:51–58. doi: 10.1007/s10557-015-6571-z. [PubMed] [CrossRef] [Google Scholar]
- Fang EF, Kassahun H, Croteau DL, Scheibye-Knudsen M, Marosi K, Lu H, Shamanna RA, Kalyanasundaram S, Bollineni RC, Wilson MA, Iser WB, Wollman BN, Morevati M, Li J, Kerr JS, Lu Q, Waltz TB, Tian J, Sinclair DA, Mattson MP, Nilsen H, Bohr VA. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metabolism. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Feng Z, Hanson RW, Berger NA, Trubitsyn A. Reprogramming of energy metabolism as a driver of aging. Oncotarget. 2016;7:15410–15420. doi: 10.18632/oncotarget.7645. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH. DNA damage and ageing: new-age ideas for an age-old problem. Nature Cell Biology. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ghosh S, Liu B, Wang Y, Hao Q, Zhou Z. lamin a is an endogenous SIRT6 activator and promotes SIRT6-Mediated DNA repair. Cell Reports. 2015;13:1396–1406. doi: 10.1016/j.celrep.2015.10.006. [PubMed] [CrossRef] [Google Scholar]
- Guleria A, Chandna S. ATM kinase: Much more than a DNA damage responsive protein. DNA Repair. 2016;39:1–20. doi: 10.1016/j.dnarep.2015.12.009. [PubMed] [CrossRef] [Google Scholar]
- Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. PNAS. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hasty P. The impact of DNA damage, genetic mutation and cellular responses on cancer prevention, longevity and aging: observations in humans and mice. Mechanisms of Ageing and Development. 2005;126:71–77. doi: 10.1016/j.mad.2004.09.036. [PubMed] [CrossRef] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Research. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [PubMed] [CrossRef] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. New England Journal of Medicine. 2009b;361:1475–1485. doi: 10.1056/NEJMra0804615. [PubMed] [CrossRef] [Google Scholar]
- Hoeijmakers JHJ. DNA damage, aging, and cancer. New England Journal of Medicine. 2009a;361:1475–1485. doi: 10.1056/NEJMra0804615. [PubMed] [CrossRef] [Google Scholar]
- Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J. The metabolic footprint of aging in mice. Scientific Reports. 2011;1:134. doi: 10.1038/srep00134. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [PubMed] [CrossRef] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [PubMed] [CrossRef] [Google Scholar]
- Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Research. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [PubMed] [CrossRef] [Google Scholar]
- Krajewski WA. Alterations in the internucleosomal DNA helical twist in chromatin of human erythroleukemia cells in vivo influences the chromatin higher-order folding. FEBS Letters. 1995;361:149–152. doi: 10.1016/0014-5793(95)00144-X. [PubMed] [CrossRef] [Google Scholar]
- Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X, Zhou Z. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. PNAS. 2011;108:12325–12330. doi: 10.1073/pnas.1102789108. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nature Reviews Molecular Cell Biology. 2015;16:393–405. doi: 10.1038/nrm4007. [PubMed] [CrossRef] [Google Scholar]
- Krüger A, Ralser M. ATM is a redox sensor linking genome stability and carbon metabolism. Science Signaling. 2011;4:pe17. doi: 10.1126/scisignal.2001959. [PubMed] [CrossRef] [Google Scholar]
- Lebel M, Spillare EA, Harris CC, Leder P. The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. Journal of Biological Chemistry. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [PubMed] [CrossRef] [Google Scholar]
- Lenaz G, D'Aurelio M, Merlo Pich M, Genova ML, Ventura B, Bovina C, Formiggini G, Parenti Castelli G. Mitochondrial bioenergetics in aging. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2000;1459:397–404. doi: 10.1016/S0005-2728(00)00177-8. [PubMed] [CrossRef] [Google Scholar]
- Li B, Comai L. Functional interaction between Ku and the werner syndrome protein in DNA end processing. Journal of Biological Chemistry. 2000;275:28349–28352. doi: 10.1074/jbc.C000289200. [PubMed] [CrossRef] [Google Scholar]
- Li Z, Zhang W, Chen Y, Guo W, Zhang J, Tang H, Xu Z, Zhang H, Tao Y, Wang F, Jiang Y, Sun FL, Mao Z. Impaired DNA double-strand break repair contributes to the age-associated rise of genomic instability in humans. Cell Death & Differentiation. 2016;23:1765–1777. doi: 10.1038/cdd.2016.65. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Pei D, Pendas AM, Cadiñanos J, López-Otín C, Tse HF, Hutchison C, Chen J, Cao Y, Cheah KS, Tryggvason K, Zhou Z. Genomic instability in laminopathy-based premature aging. Nature Medicine. 2005;11:780–785. doi: 10.1038/nm1266. [PubMed] [CrossRef] [Google Scholar]
- Liu B, Wang Z, Ghosh S, Zhou Z. Defective ATM-Kap-1-mediated chromatin remodeling impairs DNA repair and accelerates senescence in progeria mouse model. Aging Cell. 2013a;12:316–318. doi: 10.1111/acel.12035. [PubMed] [CrossRef] [Google Scholar]
- Liu B, Wang Z, Zhang L, Ghosh S, Zheng H, Zhou Z. Depleting the methyltransferase Suv39h1 improves DNA repair and extends lifespan in a progeria mouse model. Nature Communications. 2013b;4:1868. doi: 10.1038/ncomms2885. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [PubMed] [CrossRef] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [PubMed] [CrossRef] [Google Scholar]
- Luo B, Groenke K, Takors R, Wandrey C, Oldiges M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. Journal of Chromatography A. 2007;1147:153–164. doi: 10.1016/j.chroma.2007.02.034. [PubMed] [CrossRef] [Google Scholar]
- MacRae SL, Croken MM, Calder RB, Aliper A, Milholland B, White RR, Zhavoronkov A, Gladyshev VN, Seluanov A, Gorbunova V, Zhang ZD, Vijg J. DNA repair in species with extreme lifespan differences. Aging. 2015;7:1171–1182. doi: 10.18632/aging.100866. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Maslov AY, Vijg J. Genome instability, cancer and aging. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790:963–969. doi: 10.1016/j.bbagen.2009.03.020. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-Term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metabolism. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Research Reviews. 2013;12:661–684. doi: 10.1016/j.arr.2012.02.001. [PubMed] [CrossRef] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng H-L, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006a;124:315–329. doi: 10.1016/j.cell.2005.11.044. [PubMed] [CrossRef] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006b;124:315–329. doi: 10.1016/j.cell.2005.11.044. [PubMed] [CrossRef] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology. 2003;5:741–747. doi: 10.1038/ncb1024. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Paull TT. Mechanisms of ATM Activation. Annual Review of Biochemistry. 2015;84:711–738. doi: 10.1146/annurev-biochem-060614-034335. [PubMed] [CrossRef] [Google Scholar]
- Pendás AM, Zhou Z, Cadiñanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodríguez F, Tryggvason K, López-Otín C. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nature Genetics. 2002;31:94–99. doi: 10.1038/ng871. [PubMed] [CrossRef] [Google Scholar]
- Piaceri I, Bagnoli S, Tedde A, Sorbi S, Nacmias B. Ataxia-telangiectasia mutated (ATM) genetic variant in Italian centenarians. Neurological Sciences. 2013;34:573–575. doi: 10.1007/s10072-012-1188-5. [PubMed] [CrossRef] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Razani B, Feng C, Semenkovich CF. p53 is required for chloroquine-induced atheroprotection but not insulin sensitization. Journal of Lipid Research. 2010;51:1738–1746. doi: 10.1194/jlr.M003681. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Samper E, Nicholls DG, Melov S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell. 2003;2:277–285. doi: 10.1046/j.1474-9728.2003.00062.x. [PubMed] [CrossRef] [Google Scholar]
- Sarre A, Gabrielli J, Vial G, Leverve XM, Assimacopoulos-Jeannet F. Reactive oxygen species are produced at low glucose and contribute to the activation of AMPK in insulin-secreting cells. Free Radical Biology and Medicine. 2012;52:142–150. doi: 10.1016/j.freeradbiomed.2011.10.437. [PubMed] [CrossRef] [Google Scholar]
- Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metabolism. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [PubMed] [CrossRef] [Google Scholar]
- Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Research. 2004;64:2627–2633. doi: 10.1158/0008-5472.CAN-03-0846. [PubMed] [CrossRef] [Google Scholar]
- Sebastián C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–1199. doi: 10.1016/j.cell.2012.10.047. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/S0092-8674(00)00046-5. [PubMed] [CrossRef] [Google Scholar]
- Shiloh Y, Lederman HM. Ataxia-telangiectasia (A-T): An emerging dimension of premature ageing. Ageing Research Reviews. 2017;33:76–88. doi: 10.1016/j.arr.2016.05.002. [PubMed] [CrossRef] [Google Scholar]
- Shimizu I, Yoshida Y, Suda M, Minamino T. DNA damage response and metabolic disease. Cell Metabolism. 2014;20:967–977. doi: 10.1016/j.cmet.2014.10.008. [PubMed] [CrossRef] [Google Scholar]
- Shoelson SE. Banking on ATM as a new target in metabolic syndrome. Cell Metabolism. 2006;4:337–338. doi: 10.1016/j.cmet.2006.10.009. [PubMed] [CrossRef] [Google Scholar]
- Soerensen M, Dato S, Tan Q, Thinggaard M, Kleindorp R, Beekman M, Jacobsen R, Suchiman HE, de Craen AJ, Westendorp RG, Schreiber S, Stevnsner T, Bohr VA, Slagboom PE, Nebel A, Vaupel JW, Christensen K, McGue M, Christiansen L. Human longevity and variation in GH/IGF-1/insulin signaling, DNA damage signaling and repair and pro/antioxidant pathway genes: cross sectional and longitudinal studies. Experimental Gerontology. 2012;47:379–387. doi: 10.1016/j.exger.2012.02.010. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Szatmári-Tóth M, Kristóf E, Veréb Z, Akhtar S, Facskó A, Fésüs L, Kauppinen A, Kaarniranta K, Petrovski G. Clearance of autophagy-associated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration. Cell Death & Disease. 2016;7:e2367. doi: 10.1038/cddis.2016.133. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle. 2006;5:1940–1945. doi: 10.4161/cc.5.17.3191. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Tasselli L, Zheng W, Chua KF. SIRT6: novel mechanisms and links to aging and disease. Trends in Endocrinology & Metabolism. 2017;28:168–185. doi: 10.1016/j.tem.2016.10.002. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Thirumurthi U, Shen J, Xia W, LaBaff AM, Wei Y, Li CW, Chang WC, Chen CH, Lin HK, Yu D, Hung MC. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Science Signaling. 2014;7:ra71. doi: 10.1126/scisignal.2005076. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Varela I, Cadiñanos J, Pendás AM, Gutiérrez-Fernández A, Folgueras AR, Sánchez LM, Zhou Z, Rodríguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, López-Otín C. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019. [PubMed] [CrossRef] [Google Scholar]
- Vijg J. Somatic mutations, genome mosaicism, cancer and aging. Current Opinion in Genetics & Development. 2014;26:141–149. doi: 10.1016/j.gde.2014.04.002. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Whelan KA, Merves JF, Giroux V, Tanaka K, Guo A, Chandramouleeswaran PM, Benitez AJ, Dods K, Que J, Masterson JC, Fernando SD, Godwin BC, Klein-Szanto AJ, Chikwava K, Ruchelli ED, Hamilton KE, Muir AB, Wang ML, Furuta GT, Falk GW, Spergel JM, Nakagawa H. Autophagy mediates epithelial cytoprotection in eosinophilic oesophagitis. Gut. 2017;66:1197–1207. doi: 10.1136/gutjnl-2015-310341. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- White RR, Vijg J. Do DNA double-strand breaks drive aging? Molecular Cell. 2016;63:729–738. doi: 10.1016/j.molcel.2016.08.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- WHO . World Health Organ Tech Rep Ser. 2015. The Selection and Use of Essential Medicines; pp. 1–546. [PubMed] [Google Scholar]
- Wu F, Wei X, Wu Y, Kong X, Hu A, Tong S, Liu Y, Gong F, Xie L, Zhang J, Xiao J, Zhang H. Chloroquine promotes the recovery of acute spinal cord injury by inhibiting autophagy-associated inflammation and endoplasmic reticulum stress. Journal of Neurotrauma. 2018 doi: 10.1089/neu.2017.5414. [PubMed] [CrossRef] [Google Scholar]
- Yang YP, Hu LF, Zheng HF, Mao CJ, Hu WD, Xiong KP, Wang F, Liu CF. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacologica Sinica. 2013;34:625–635. doi: 10.1038/aps.2013.5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [PubMed] [CrossRef] [Google Scholar]
- Zhang YW, Otterness DM, Chiang GG, Xie W, Liu YC, Mercurio F, Abraham RT. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Molecular Cell. 2005;19:607–618. doi: 10.1016/j.molcel.2005.07.019. [PubMed] [CrossRef] [Google Scholar]
- Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhou N, Xiao H, Li TK, Nur-E-Kamal A, Liu LF. DNA damage-mediated apoptosis induced by selenium compounds. Journal of Biological Chemistry. 2003;278:29532–29537. doi: 10.1074/jbc.M301877200. [PubMed] [CrossRef] [Google Scholar]
- eLife. 2018; 7: e34836. »
- Decision letter
Decision letter
In the interests of transparency, eLife includes the editorial decision letter and accompanying author responses. A lightly edited version of the letter sent to the authors after peer review is shown, indicating the most substantive concerns; minor comments are not usually included.
Thank you for submitting your article "Boosting ATM Activity Promotes Longevity in Nematodes and Mice" for consideration by eLife. Your article has been favorably evaluated by Jessica Tyler (Senior Editor) and three reviewers, one of whom, Matt Kaeberlein (Reviewer #1), is a member of our Board of Reviewing Editors.
The reviewers have discussed the reviews with one another and the Reviewing Editor has drafted this decision to help you prepare a revised submission.
Summary:
All of the reviewers recognized the important and novel insights provided in this manuscript linking ATM, SIRT6, and longevity, but that there are some key revisions that need to be made before publication. In particular, the effects reported from CQ treatment are quite interesting, but could be due to targets other than ATM. It would greatly strengthen the manuscript to show genetically that ATM activation/overexpression recapitulates at least some of the reported phenotypes. This may not be feasible in mice, but is doable in worms or cells.
Several additional controls are needed to demonstrate phosphorylation of SIRT6 by ATM, as noted by reviewer #2.
There is some question as to whether the increases in lifespan observed in the manuscript are due to improved DNA damage response or to metabolic changes. In the absence of additional data addressing these two possibilities, the authors are encouraged to interpret their data in a more agnostic way and consider both models. This should be done both in the text and in a revised model figure.
Use of different cell lines for different experiments is confusing and detracts from confidence in the results. For example, why were MEFs used for senescence but HSFs were used for other assays, and HepG2 for even other assays. Optimally, most or all of the experiments would be done in a single consistent cell line.
There are numerous errors in grammar and data presentation throughout the manuscript. Several of these are listed below, and the authors should take much more care in preparing the revised version of their manuscript. Careful proofreading for English sentence structure and phrasing is also important.
Essential revisions:
1) Title: The title is a bit misleading and implies that the study shows that boosting ATM activity promotes longevity in wild type mice. I don't think it's necessary for the authors to show lifespan extension in wild type mice for publication, but their title and Abstract need to be changed so that it is clear that the longevity effects in mice are limited to short-lived mouse models of disease.
2) The authors should explain why the replicative senescence experiments were done in MEFs rather than HSFs and perhaps repeat these experiments in HSFs. Did the experiment not work in HSFs? Showing that CQ can extend replicative lifespan in HSFs would seem to be more relevant than MEFs. I also worry that the replicative capacity of the MEFs here is so short. Was this done under atmospheric oxygen conditions and, if so, it needs to be repeated under lower oxygen.
3) I found the worm experiments to be the least convincing part of the manuscript. Drug studies in worms are complicated by potential effects on the bacterial food, which I don't think was addressed here. There is no biochemical evidence that CQ gets into worms or modifies activity of the worm ATM homolog. There is no evidence that the worm ATM homolog phosphorylates the worm SIRT6 homolog. Again, the story sticks together with the model, but there are a lot of apparent assumptions that need to be made here. I realize the tools aren't available to do some of this, but you could show that transgenic overexpression of ATM is sufficient to have effects similar to CQ and increase lifespan in a sir-2.4-dependent manner.
4) The part of the paper dealing with phosphorylation of SIRT6 by ATM is novel and interesting, but needs some additional controls.
a) Figure 3D shows that both SIRT6 and ATM are in the nucleus (not surprisingly), but it is hard to judge whether they are co-localized. Ether a higher resolution confocal microscope is needed, or the figure can be removed.
b) Figure 4C, D: the gel does not show any difference between SIRT6 WT and S112D, yet quantification on the right shows very large effect. The authors need to provide a gel that corresponds to the quantification.
c) It is important to knock-in the SIRT6 S112 mutations in a cell line and perform RNAseq (or qRT-PCR on glycolytic genes) to prove that the mutation indeed affects SIRT6 regulated genes.
5) In Figure 2—figure supplement 1C, Figure 2h and Figure 2—figure supplement 2B, there were not have enough biological replicates.
- eLife. 2018; 7: e34836. »
- Author response
Author response
Essential revisions:
1) Title: The title is a bit misleading and implies that the study shows that boosting ATM activity promotes longevity in wild type mice. I don't think it's necessary for the authors to show lifespan extension in wild type mice for publication, but their title and Abstract need to be changed so that it is clear that the longevity effects in mice are limited to short-lived mouse models of disease.
We appreciate the reviewers’ suggestion. We have now removed related data in the revised manuscript and corresponding figures as requested. We will investigate the effect of CQ on physiological ageing in an independent project. As suggested, we changed the title to “Boosting ATM activity alleviates ageing and extends lifespan in a mouse model of progeria”. Accordingly, the Abstract was re-written; the figure panels were reorganized (see revised Figure 5 and Figure 5—figure supplement 1).
2) The authors should explain why the replicative senescence experiments were done in MEFs rather than HSFs and perhaps repeat these experiments in HSFs. Did the experiment not work in HSFs? Showing that CQ can extend replicative lifespan in HSFs would seem to be more relevant than MEFs. I also worry that the replicative capacity of the MEFs here is so short. Was this done under atmospheric oxygen conditions and, if so, it needs to be repeated under lower oxygen.
We thank the reviewers for their comments and suggestions. We performed the requested experiments in HSFs and showed consistent result that CQ extends replicative lifespan (see revised Figure 1J). We did notice that MEFs have a very limited growth capacity and could be spontaneously immortalized at a low frequency as a result of prolonged passaging (Sun and Taneja, 2007). Indeed, to demonstrate that ATM declines with age and senescence, we utilized two cell lines, HSFs and MEFs (revised Figure 1A and 1B). We showed that activating ATM via CQ retards senescence in both HSFs and MEFs (revised Figure 1H, 1I and Figure 1—figure supplement 2D-E). Under atmospheric oxygen conditions, normal primary MEFs undergo 10 ± 2 population doublingsin vitro, and then rapidly enter a slow growth status that has been described as replicative senescence (Blasco et al., 1997; Rittling, 1996; Todaro and Green, 1963). As suggested, we examined the effect of CQ on senescence of MEFS at physiological (3%) oxygen level. Compared with 20% O2, MEFs in 3% O2 exhibit no remarkable signs of senescence even reaching passage 9 (see Author Response Image 1), which is indeed consistent with existing literature (Parrinello et al., 2003). As indicated, the cultures in 3% O2 can reach more than 60 population doublings without any senescence phenotypes. In current project, to address that CQ treatment alleviates senescence by activating ATM and promoting clearance of DNA damage, we think it is appropriate to examine the replicative lifespan of MEFs under standard culture conditions, which exhibit shortened lifespan predominantly triggered by DNA damage accumulation. Thus, the Author Response Image 1 was not included in the revised manuscript.
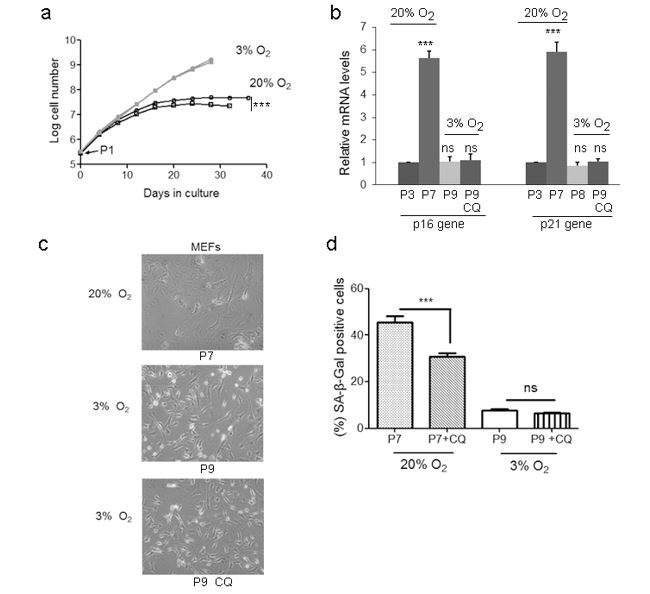
(a) MEFs at passage 1 were cultured in 20% O2 (black) or 3% O2 (grey) with treatment of 1 μM CQ (circles) or DMSO (squares), and cell number was determined at each passage. ***P < 0.01. (b) Quantitative RT-PCR analysis of mRNA levels of p16 and p21 genes in different passages of MEFs with or without treatment of CQ in 20% O2 or 3% O2. ***P < 0.001. ‘ns’ indicates no significant difference. (c) Representative images showing morphology of MEFs under the indicated culture conditions with or without CQ treatment. (d) Quantification of SA-β-Gal-positive cells under indicated culture conditions. Data represent the means ± SEM. ***P < 0.001; ‘ns’ indicates no significant difference.
3) I found the worm experiments to be the least convincing part of the manuscript. Drug studies in worms are complicated by potential effects on the bacterial food, which I don't think was addressed here. There is no biochemical evidence that CQ gets into worms or modifies activity of the worm ATM homolog. There is no evidence that the worm ATM homolog phosphorylates the worm SIRT6 homolog. Again, the story sticks together with the model, but there are a lot of apparent assumptions that need to be made here. I realize the tools aren't available to do some of this, but you could show that transgenic overexpression of ATM is sufficient to have effects similar to CQ and increase lifespan in a sir-2.4-dependent manner.
We agree with the reviewers that drug studies in worms are complicated and there is lack of solid evidence to support that worm ATM could phosphorylate sir2.4. In this study, we performed CQ treatment on N2, atm-1 KO and sir2.4-depleted nematodes, showing that CQ extends lifespan of N2 worms, but not that of atm-1 KO or sir2.4-depleted worms. The parallel treatment largely precludes potential effects of CQ on the bacterial food. To further strengthen the conclusion, as the reviewers suggested, we attempted to investigate the lifespan-extending effect of extra copies of ATM-1 in worms. Since the endogenous AMT-1 gene promoter is unknown and a 2 kb region at the upstream of ATM-1 ORF did not work in our test, we constructed a large plasmid pPD95_75 with GFP-tagged full-length ATM-1 (~7kbp) under the promoter of sir2.4 (Chiang et al., 2012). The results indeed showed significant effect on lifespan extension (see Author Response Image 2). Of note, the heterozygous instead of homozygous status of a SNP, albeit both enhance the transcription of ATM, is associated with longevity in Chinese and Italian populations (Chen et al., 2010; Piaceri et al., 2013). However, though GFP tag was detected, we were unable to determine the amount of ectopic ATM-1 in transgenic worms. In future study, it would be worthwhile to evaluate how many extra copies of Atm could maximally promote longevity in model organisms.
For dosage-dependent studies, while both 10 μM and 1 μM of CQ activate ATM, only 1 μM of CQ was suitably applied in the studies, which promotes longevity in HSFs, Zmpste24-/- mice, and nematodes, but exhibits little effect in ATM-depleted mice and worms. Perhaps it is an important facet of the amount of ATM for pro-longevity, requiring further study. We discussed this in the revised manuscript.
C. elegans with conserved DDR mechanism seems to be as a promising model for the investigation of DNA repair (Jones et al., 2012; Lemmens and Tijsterman, 2011). ATM-1 in C. elegans, the homolog of mammalian ATM, is known to function in DDR and DSB repair, and its activity and functional interactions are conserved between worms and humans (Lee et al., 2010; Lemmens and Tijsterman, 2011). Similar to A-T patients and Atm-/- mice, loss-of-function of ATM-1 results in genomic instability and shortened lifespan (Fang et al., 2016; Jones et al., 2012). To test the lifespan-extending effect of ATM at organismal level, we employed Zmpste24-/- mice with reduced ATM and DNA repair capacity, ATM-/- mice and wild-type nematodes for CQ administration. The results showed that CQ improves SIRT6 protein level and extends the lifespan of Zmpste24-/- mice and N2 worms, but not that of Atm-/- mice and ATM-1 KO worms. Moreover, the cellular data indicated that CQ promotes replicative lifespan of HSFs in an ATM-dependent manner. Biochemical studies revealed that ATM phosphorylates SIRT6 at the conserved Ser112 residue, blocking MDM2-mediated proteasomal degradation. Generally, based on these studies, we rationally believe that our current data is adequate to support a pro-longevity role of ATM.
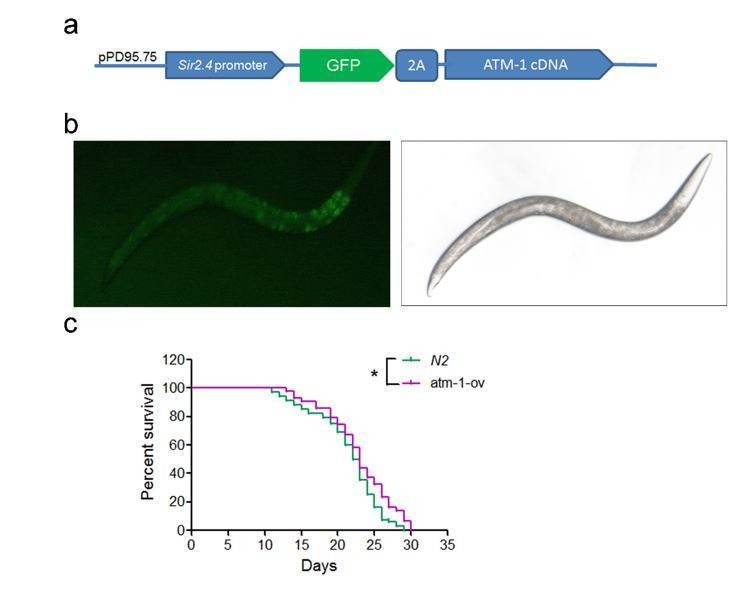
(a) The strategy for constructing ATM-1 overexpressing plasmid with GFP-tag. (b) Representative images showing the detectable GFP expression in worms. (c) Survival analysis of worm line expressing GFP-ATM-1. *P < 0.05.
4) The part of the paper dealing with phosphorylation of SIRT6 by ATM is novel and interesting, but needs some additional controls.
a) Figure 3D shows that both SIRT6 and ATM are in the nucleus (not surprisingly), but it is hard to judge whether they are co-localized. Ether a higher resolution confocal microscope is needed, or the figure can be removed.
We thank the reviewers for the suggestion. SIRT6 is a nuclear NAD+-dependent deacetylase; ATM kinase is highly enriched in nuclei and also detectable in cytoplasm (Gately et al., 1998), which are consistent with our immunostaining result using SIRT6 and ATM antibodies. We have now presented high resolution confocal images, clearly showing intracellular punctate co-localization of SIRT6 and ATM (revised Figure 3D).
b) Figure 4C, D: the gel does not show any difference between SIRT6 WT and S112D, yet quantification on the right shows very large effect. The authors need to provide a gel that corresponds to the quantification.
We are thankful to the reviewers for their careful reading. We now presented new Western blots in revised Figure 4C, showing more clearly that S112D significantly retards SIRT6 degradation.
c) It is important to knock-in the SIRT6 S112 mutations in a cell line and perform RNAseq (or qRT-PCR on glycolytic genes) to prove that the mutation indeed affects SIRT6 regulated genes.
We appreciate for the reviewers’ insightful comments. Deficiency of ATM or SIRT6 enhances the expression of glycolytic genes, whereas re-expressed SIRT6 in ATM-depleted HepG2 rescues the increase of glycolysis. ATM directly interacts with SIRT6 and phosphorylates it at Ser112. It is important to check the inhibitory effect of SIRT6 S112 mutations on glycolytic gene expression. Unfortunately, we failed to knock-in the SIRT6 S112 mutations in HepG2 by CRISPR-Cas9 system. Instead, to address this issue, we have now provided additional evidence from a practical approach. We utilized SIRT6-depleted HepG2 cells (revised Figure 3—figure supplement 1L), generated by shRNA lentiviral system (see Materials and methods) and re-constituted with SIRT6 WT, S112A and S112D. As shown in revised Figure 3M, the qRT-PCR data demonstrated that SIRT6 S112D exhibits more inhibitory effect on glycolytic gene expression than S112A. In addition, sub-cellular fractionation experiment showed that S112D enhances the chromatin-binding capacity of SIRT6, whereas S112A abrogated it (see revised Figure 3N). Our data suggest that SIRT6 S112A loses the regulation on targeted genes due to reduced chromatin-binding affinity and protein stability. Additional data indicate that ATM depletion or S112A mutation promotes SIRT6 degradation via MDM2-mediated proteasomal pathway.
5) In Figure 2—figure supplement 1C, Figure 2h and Figure 2—figure supplement 2B, there were not have enough biological replicates.
According to the reviewer’s suggestion, we have done these experiments with more repeats (n = 6 for each group) and did statistical analysis as shown in revised Figure 2—figure supplement 1C, Figure 2H and Figure 2—figure supplement 2B. The details have been included in revised figure legends.