- Journal List
- eLife
- PMC6287945

A whole lifespan mouse multi-tissue DNA methylation clock
Margarita V Meer
1Division of Genetics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, United States
Dmitriy I Podolskiy
1Division of Genetics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, United States
Alexander Tyshkovskiy
1Division of Genetics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, United States
2Center for Data-Intensive Biomedicine and Biotechnology, Skolkovo Institute of Science and Technology, Moscow, Russia
Vadim N Gladyshev
1Division of Genetics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, United States
Associated Data
- Data Citations
- Meer MV, Podolskiy DI, Tyshkovskiy AE, Gladyshev VN. 2018. DNA methylation of mouse tissues. NCBI Gene Expression Omnibus. GSE121141
- Stubbs TM, von Meyenn F, Katrien-Stark A, Krueger F, Reik W. 2017. Multi-tissue DNA methylation age predictor in mouse. GEO. GSE93957
- Reizel Y, Spiro A, Sabag O, Skversky Y, Hecht M, Keshet I, Berman BP, Cedar H. 2014. Gender-specific post-natal demethylation and establishment of epigenetic memory. GEO. GSE60012
- Petkovich DA, Podolskiy DI, Lobanov AV, Gladyshev VN. 2016. Using DNA methylation profiling to evaluate biological age and longevity interventions. GEO. GSE80672
- Cannon MV, Buchner DA, Hester J, Miller H, Sehayek E, Nadeau JH, Serre D. 2013. Maternal Nutrition Induces Pervasive Gene Expression Changes but no Detectable DNA Methylation Differences in the Liver of Adult Offspring [RRBS] GEO. GSE52266
- Zhang C, Edepli KS, Lujambio A. 2016. Genome-wide DNA methylation profiling of mouse liver. GEO. GSE80761
- Schillebeeckx M, Mitra RD. 2013. Laser Capture Microdissection-Reduced Representation Bisulfite Sequencing (LCM-RRBS) maps changes in DNA methylation associated with gonadectomy-induced adrenocortical neoplasia in the mouse. GEO. GSE45361
- Cole JJ, Robertson NA, Rather MI, Adams PD. 2016. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions (WGBS 1) GEO. GSE89273
- Cole JJ, Robertson NA, Rather MI, Adams PD. 2016. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions (WGBS 2) GEO. GSE89274
- McCormick H, Hur s, Young P, Cropley J, Booher K, Cheung H, Giannolatou E, Suter C. 2016. Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. GEO. GSE84573
- Supplementary Materials
- Supplementary file 2: Information on samples sequenced by RRBS in the study and included in the analysis.elife-40675-supp2.xlsx (39K)DOI: 10.7554/eLife.40675.021Supplementary file 4: Genes overlapping in pairs of clocks.elife-40675-supp4.xlsx (34K)DOI: 10.7554/eLife.40675.023Supplementary file 5: Distribution of samples across libraries.elife-40675-supp5.xlsx (33K)DOI: 10.7554/eLife.40675.024
- Data Availability Statement
Sequencing data have been deposited in GEO under accession code {"type":"entrez-geo","attrs":{"text":"GSE121141","term_id":"121141"}}GSE121141
The following dataset was generated:
Meer MV, Podolskiy DI, Tyshkovskiy AE, Gladyshev VN. 2018. DNA methylation of mouse tissues. NCBI Gene Expression Omnibus. GSE121141
The following previously published datasets were used:
Stubbs TM, von Meyenn F, Katrien-Stark A, Krueger F, Reik W. 2017. Multi-tissue DNA methylation age predictor in mouse. GEO. GSE93957
Reizel Y, Spiro A, Sabag O, Skversky Y, Hecht M, Keshet I, Berman BP, Cedar H. 2014. Gender-specific post-natal demethylation and establishment of epigenetic memory. GEO. GSE60012
Petkovich DA, Podolskiy DI, Lobanov AV, Gladyshev VN. 2016. Using DNA methylation profiling to evaluate biological age and longevity interventions. GEO. GSE80672
Cannon MV, Buchner DA, Hester J, Miller H, Sehayek E, Nadeau JH, Serre D. 2013. Maternal Nutrition Induces Pervasive Gene Expression Changes but no Detectable DNA Methylation Differences in the Liver of Adult Offspring [RRBS] GEO. GSE52266
Zhang C, Edepli KS, Lujambio A. 2016. Genome-wide DNA methylation profiling of mouse liver. GEO. GSE80761
Schillebeeckx M, Mitra RD. 2013. Laser Capture Microdissection-Reduced Representation Bisulfite Sequencing (LCM-RRBS) maps changes in DNA methylation associated with gonadectomy-induced adrenocortical neoplasia in the mouse. GEO. GSE45361
Cole JJ, Robertson NA, Rather MI, Adams PD. 2016. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions (WGBS 1) GEO. GSE89273
Cole JJ, Robertson NA, Rather MI, Adams PD. 2016. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions (WGBS 2) GEO. GSE89274
McCormick H, Hur s, Young P, Cropley J, Booher K, Cheung H, Giannolatou E, Suter C. 2016. Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. GEO. GSE84573
Abstract
Age predictors based on DNA methylation levels at a small set of CpG sites, DNAm clocks, have been developed for humans and extended to several other species. Three currently available versions of mouse DNAm clocks were either created for individual tissues or tuned toward young ages. Here, we constructed a robust multi-tissue age predictor based on 435 CpG sites, which covers the entire mouse lifespan and remains unbiased with respect to any particular age group. It can successfully detect the effects of certain lifespan-modulating interventions on DNAm age as well as the rejuvenation effect related to the transition from fibroblasts to iPSCs. We have carried out comparative analyses of available mouse DNAm clocks, which revealed their broad applicability, but also certain limitations to the use of tissue-specific and multi-tissue age predictors. Together, these tools should help address diverse questions in aging research.
Introduction
A robust and precise marker, which can be used to estimate the biological age of organisms and to evaluate the effect of different interventions on lifespan has been a Holy Grail of aging research since the first days of this field. Various biomarkers of age have previously been suggested, which are based on telomere length (Harley et al., 1990), mutation accumulation (Dollé et al., 2000; Podolskiy et al., 2016; Podolskiy and Gladyshev, 2016), gene expression levels (de Magalhães et al., 2009) or T-cell-specific DNA rearrangements (Zubakov et al., 2010). However, these approaches to construct a precise biomarker of age proved to be relatively limited in their potential to assess the aging process and examine its modulation by various interventions, mostly due to large variability of detected ages. In contrast, analyses of DNA methylation provided an opportunity for much more accurate estimation of age of organisms. This method is based on the chronological age of subjects and reflects positive or negative changes which are associated with lifespan extension or shortening. Such biomarkers do not measure the biological age in a direct way but may reveal a change in it.
Age predictors based on DNA methylation were first proposed for human saliva samples (Bocklandt et al., 2011) and were later developed into robust blood-specific (Hannum et al., 2013) and multi-tissue (Horvath, 2013) biomarkers of age. To date, age predictors based on systematic changes of DNA methylation with age, DNAm clocks, remain the most precise markers of age. For example, the human multi-tissue DNAm clock has a median error of 3.6 years (Horvath, 2013), which does not exceed ~3% of maximal human lifespan. Additional versions of human DNAm clock are being developed that can address broad medical and public health questions related to aging. The already developed human DNAm clocks reflect age acceleration associated with genetic background, such as progeria (Maierhofer et al., 2017), as well as caused by lifestyle and diet, such as smoking and obesity (Quach et al., 2017; Levine et al., 2018). Age predictors based on DNA methylation were also recently constructed for whales (Polanowski et al., 2014), wolves and dogs (Thompson et al., 2017). Availability of these clocks may help to speed up testing lifespan-extending interventions, such as rapamycin (Urfer et al., 2017). It remains unclear, however, whether the best clocks should utilize a few key CpG sites, a large group of sites or a change of the pattern of global methylation.
Application of DNAm clocks to assess the DNA methylation age would be especially useful when applied to common model organisms of aging. In this regard, three DNAm clock versions have been developed for mice, including blood-based (Petkovich et al., 2017) and liver-based (Wang et al., 2017) clocks, and a young age-specific multi-tissue clock (Stubbs et al., 2017) (Table 1). These clocks have been already used to test the effects of several genetic, dietary or pharmacological interventions (Petkovich et al., 2017; Wang et al., 2017; Stubbs et al., 2017; Hahn et al., 2017; Cole et al., 2017) on mouse lifespan. However, the existing mouse DNAm clocks have a limitation of tissue specificity, and it also remains unclear how they relate to and perform with respect to each other. Here, we created a DNAm clock based on DNA methylomes of different tissues collected from mice aged from 1 week to 35 months old. We further performed comparative analysis of previously published DNAm age estimators and the newly constructed DNAm multi-tissue clock.
Table 1.
| Tissue type | Reported precision, R2 | Age range of training set, months | Number of CpG sites in the model | Publication |
|---|---|---|---|---|
| Blood | >0.9008 | 3.–35 | 90 | (Petkovich et al., 2017) |
| Liver | 0.91 | 0.2–26 | 148* | (Wang et al., 2017) |
| Multi-tissue | 0.7 | 0.2–9.5 | 329 | (Stubbs et al., 2017) |
| Multi-tissue | 0.89 | 0.2–35 | 435 | This paper |
*Unlike other DNAm clocks, the liver clock is based on a combination of methylation levels on both strands and thus it corresponds to 296 genomic positions.
Results
Construction of a multi-tissue clock
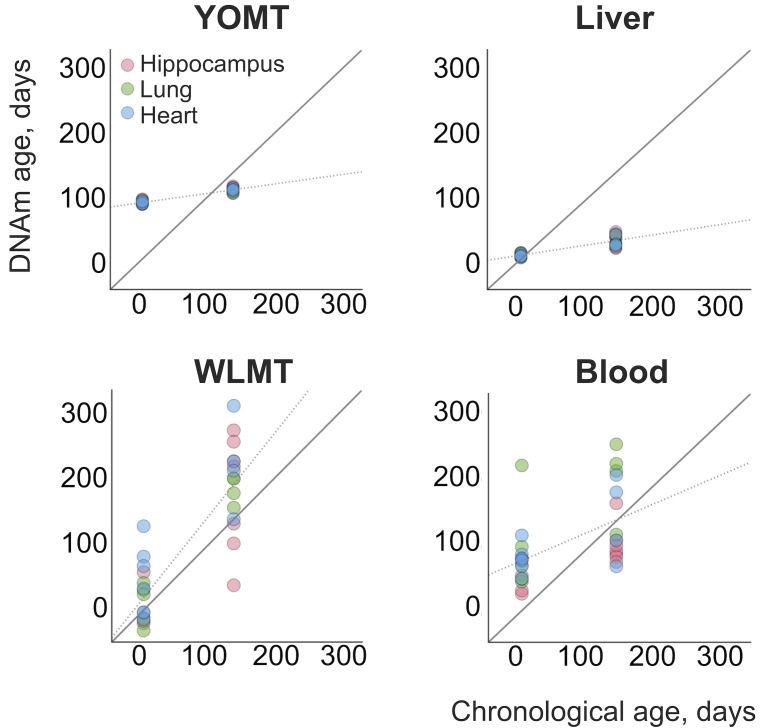
A mouse DNAm multi-tissue clock (YOung Multi-Tissue or YOMT in what follows) was recently developed (Stubbs et al., 2017), which was tuned for tissues of younger mice, for example its mean absolute error, MAE, increased from 2.14 to 4.66 weeks if applied to animals of <20 weeks and 20–40 weeks of age, respectively. In addition, application of this clock to new samples required normalization of the data to the original dataset used to construct YOMT (Stubbs et al., 2017). We aimed to create a multi-tissue age predictor, which can be easily employed by the community to estimate the DNA methylation age of samples of all accessible chronological ages (Whole Lifespan Multi-tissue or WLMT clock in what follows).
To develop the clock, we first collected publicly available data on DNA methylation in mice (Supplementary file 1). The combined dataset was strongly biased toward younger chronological ages, with the blood being the only tissue well represented by different ages. Therefore, to construct the methylation clock based on comprehensive compendium of samples, we additionally performed reduced representation bisulfite sequencing (RRBS) of 76 liver, lung, brain and heart samples of 6-, 10-, 12-, 20- and 30-month-old C57Bl/6 male mice, with 4–5 biological replicates per tissue per time point, except for two age/tissue points (Supplementary file 2).
While in theory whole genome bisulfite sequencing (WGBS) should include all CpG sites obtained by any other enrichment method, in reality the overlap between the results of WGBS and RRBS is such that only ~20% of CpG sites obtained by RRBS are sufficiently covered by WGBS if the same samples are sequenced by both methods (Harris et al., 2010). Consequently, pooling together methylation profiles obtained by the two methods resulted in too few sites covered in all pooled samples. Also, even though the use of WGBS by the research community to characterize DNA methylation becomes more common, the number of available datasets obtained for whole genomes remains relatively limited. For this reason, we used only RRBS data to construct the multi-tissue DNAm clock.
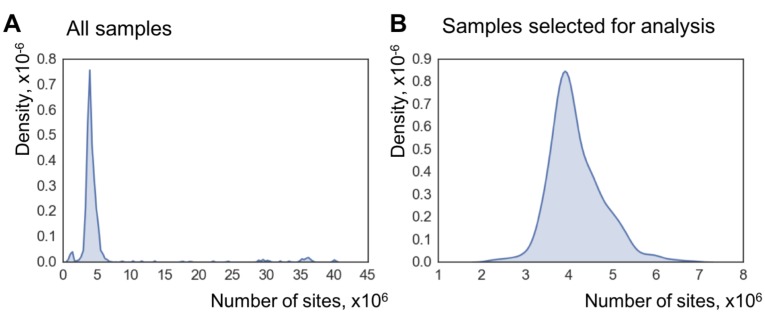
We filtered out RRBS samples with fewer than

(A) Datasets used for the clock construction and initial validation. Radii of pie charts correspond to the number of samples in a group. The range of ages is shown above (in months). Performance of the whole-lifespan multi-tissue clock on training (B) and test (C) sets. MAE on training and test sets was 28.6 and 72.7 days, respectively. Tissues used are indicated on the right according to the color scheme.
Figure 1—figure supplement 1.
(A) and in samples with covered
Figure 1—figure supplement 2.
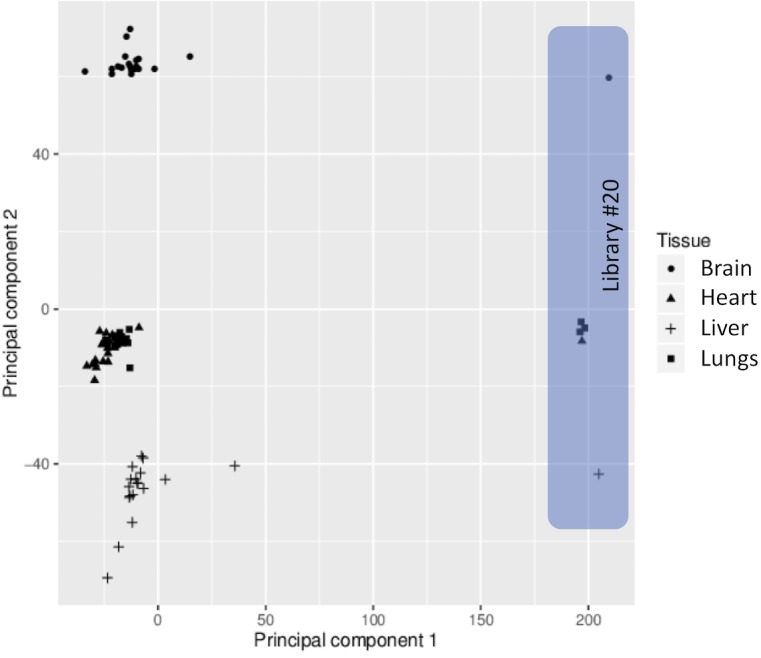
Library ‘M20’ was not used in the preparation of the clock.
Figure 1—figure supplement 3.
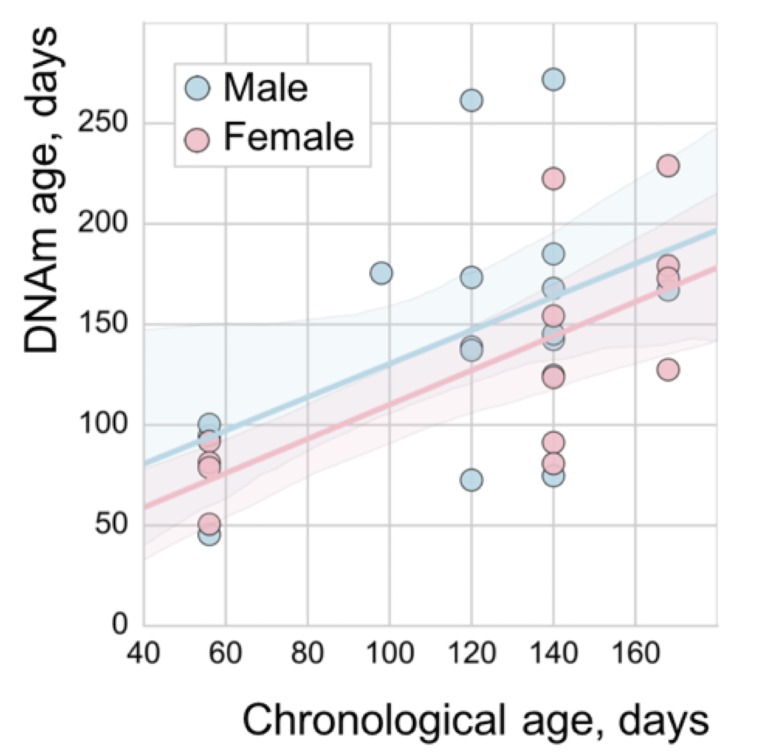
Figure 1—figure supplement 4.
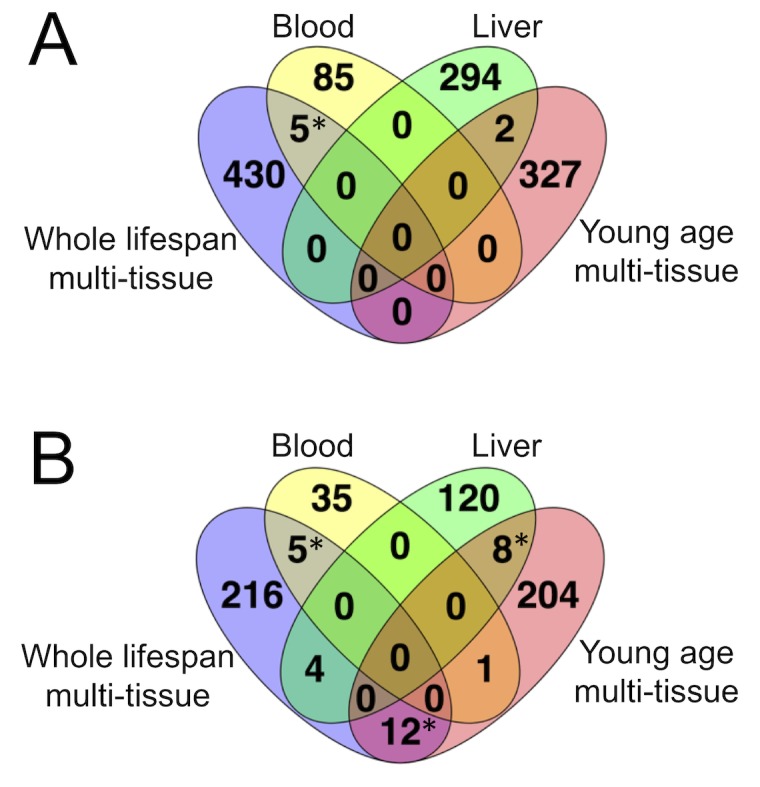
(A) overlap in CpG sites. (B) overlap in genes containing the clock sites. * - significant with
Figure 2—figure supplement 1.
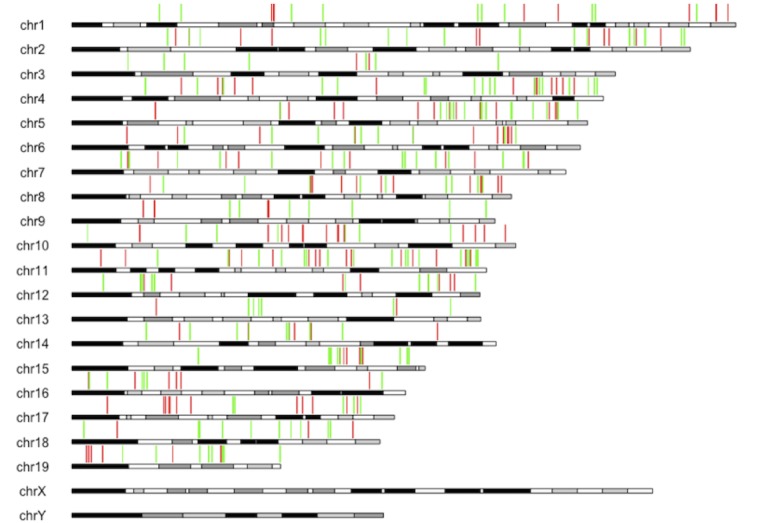
Sites with positive weights (increasing methylation with age) are shown in green, and those with negative weights (decreasing methylation with age) in red.
Figure 2—figure supplement 2.

Figure 2—figure supplement 3.
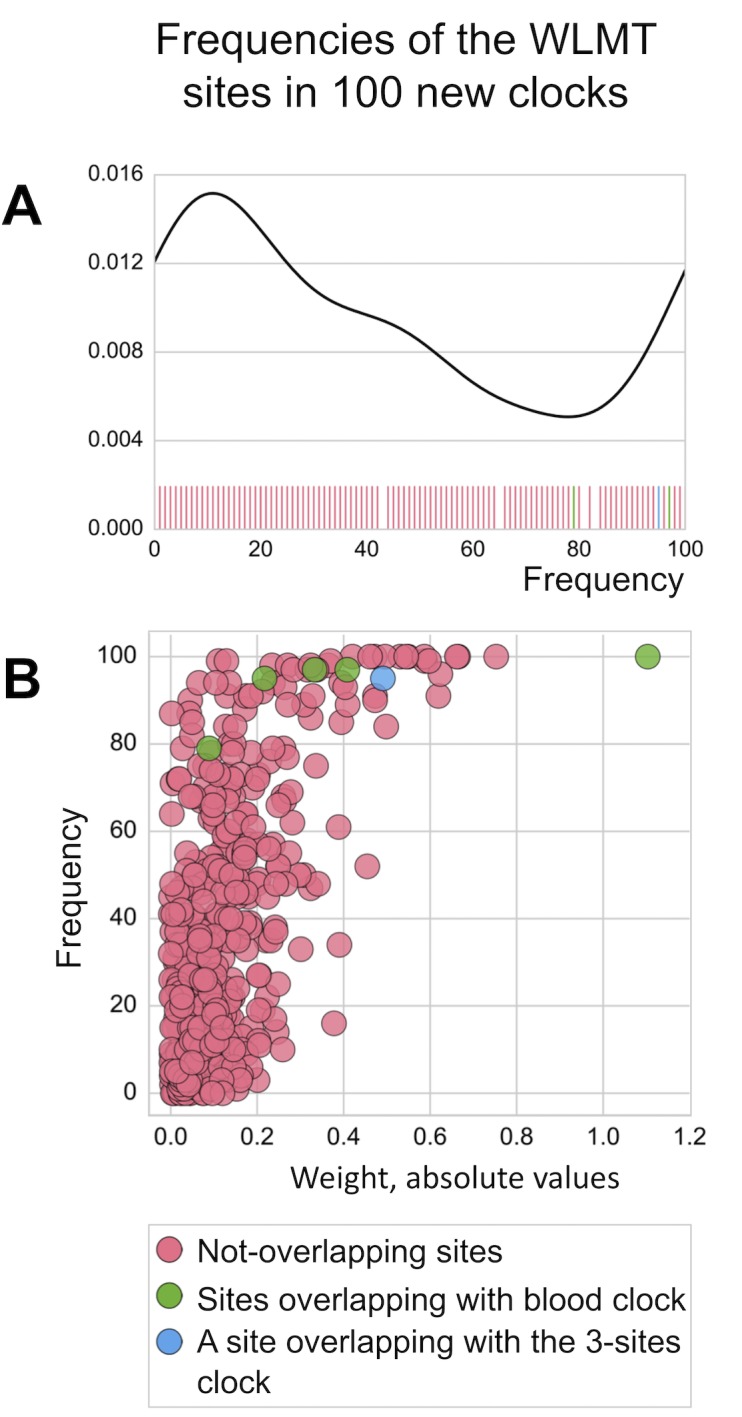
100 clocks are trained on different subsets of the set of samples used in WLMT construction. (A) Density plot showing the overall distribution of frequencies of WLMT clock sites as found in the generated 100 clocks. Carpet plot shows the exact values of frequencies. (B) Relation of the absolute values of weight of sites in the WLMT clock and frequency of these sites in the 100 alternative clocks.
Figure 2—figure supplement 4.
Tissues used in construction of the clock are shown.
Figure 2—figure supplement 5.
Figure 2—figure supplement 6.
The majority of presently available RRBS samples come from male mice. Therefore, WLMT is mostly (84%) based on male samples. We tested how this affects performance on samples of different sexes by comparison of predictions for male and female samples of the same age range (from 56 to 168 days old). MAE was lower for female samples (33 days), while it was 43.7 days for the male samples, but this difference was not significant (
Comparison of existing mouse DNAm clocks
Three different mouse DNAm clocks have previously been reported (Table 1), but their performance has not yet been comparatively analyzed. The reported MAE for YOMT was 23.3 days. MAE has not been estimated, but the clocks based on the half of the samples were estimated to have MAE of 1–3 months for young samples (<10 months old) and 5–7 months for older ages. MAE for the 22-month-old liver samples measured by the liver clock was 4.2 months.
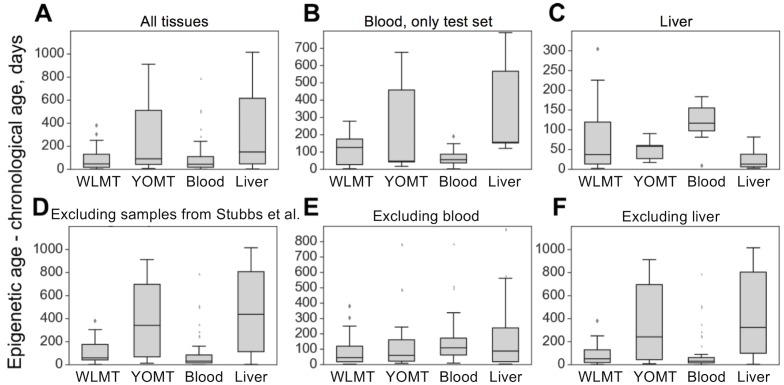
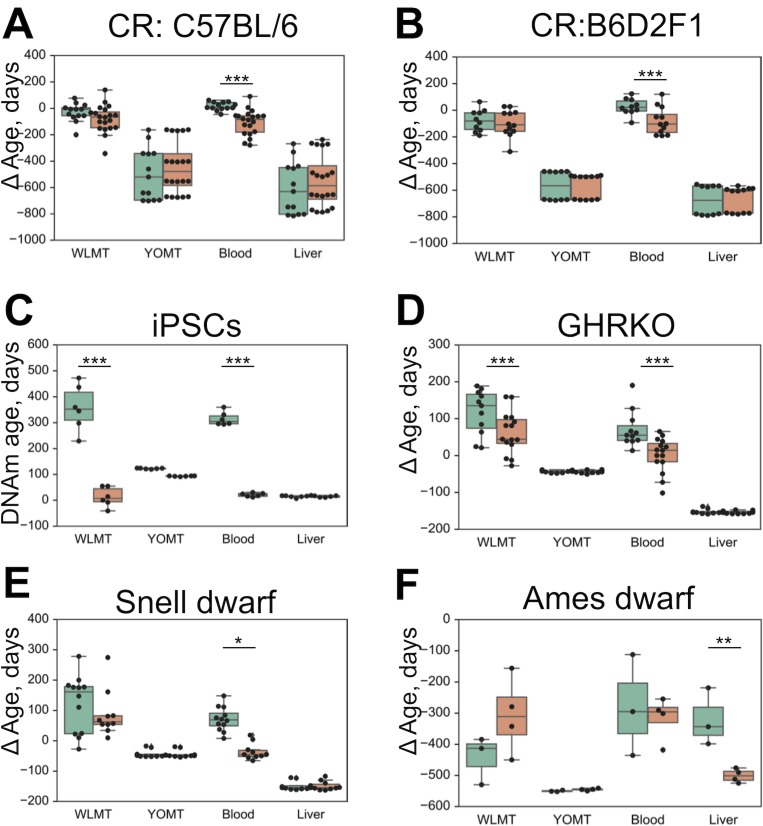
The four age predictors that are compared here to the WLMT test set (Figure 3A) are biased toward a better performance as our test set includes samples from training sets of these clocks. WLMT was based on samples representing all these datasets combined. Since RRBS results typically include batch effects/dependence on experiment location and procedure, this gives an advantage to WLMT because other clocks were tested on the datasets not represented in their original training sets. As almost all samples turned out to be included into a training set of one clock studied here or another, we utilized different subsets of samples for comparative analyses (Figure 3B-F). All untreated wild-type C57BL/6 samples were used for the construction of the blood methylation clock. Thus, it was impossible to compare the blood clock to other age estimators by applying it to the same strain without removing an associated performance bias. Instead, we used control samples from three different studies: growth hormone receptor (GHR) knockout (KO) and Snell dwarf mice as well as caloric restriction applied to B6D2F1 mice (Petkovich et al., 2017). In this setup, the methylation clock built using blood samples outperformed all other predictors (
(A) In this case, WLMT clock was applied to its test set, whereas for the other age predictors this set also included their training set samples. (B) Blood samples which were not used in any of the clocks. (C) Only liver samples. (D) Samples of all tissues, excluding those used in the YOMT clock. (E) Samples of all non-blood tissues. (F) All tissues, excluding liver.
Figure 3—figure supplement 1.
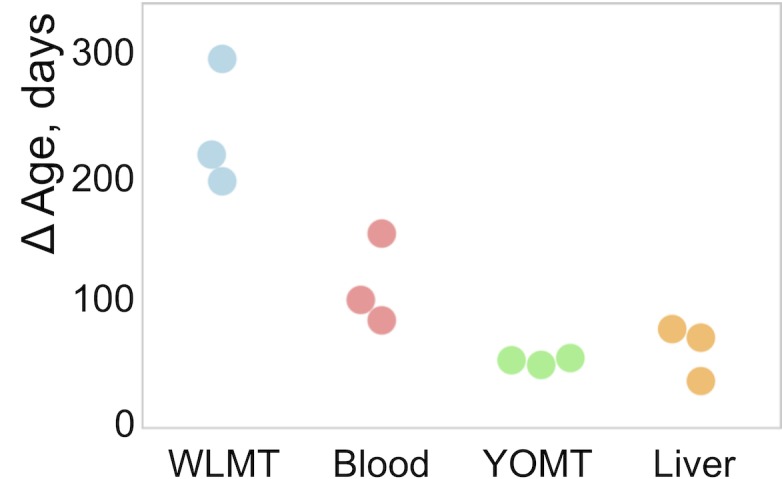
∆Age shows difference between DNAm age and chronological age. Chronological age of the included samples is 180 days. MAE is 244 days for the WLMT and 65 days for the liver clock.
Figure 3—figure supplement 2.
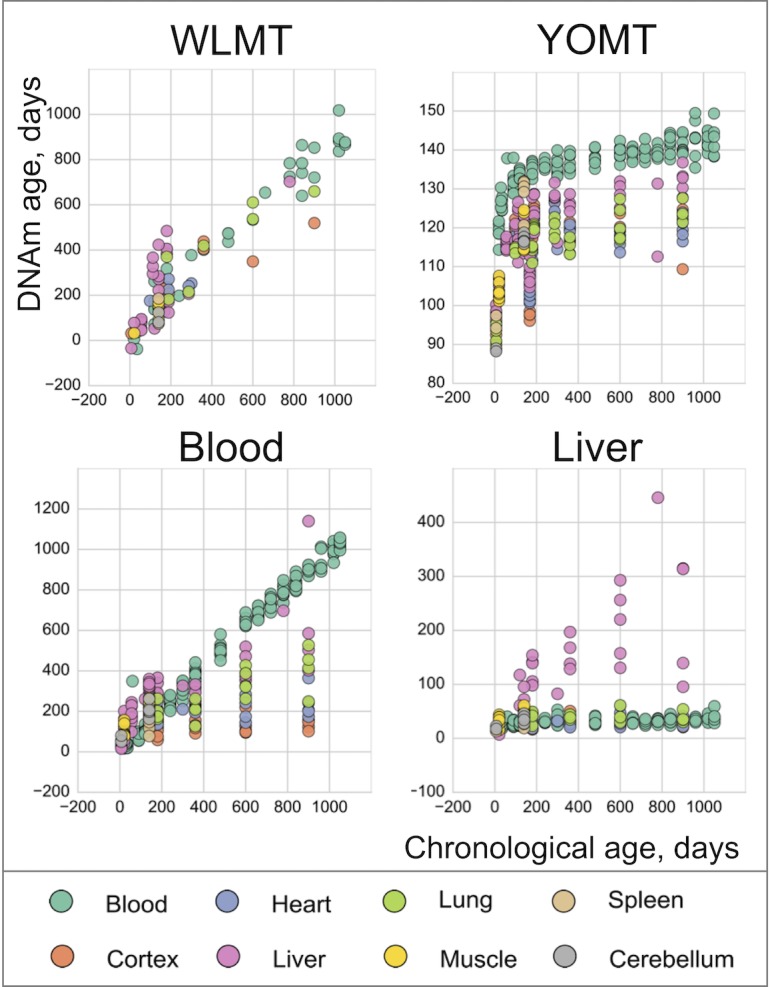
WLMT: only test samples are included. YOMT: test and training sets are shown. Blood samples have not been used in development of this clock. Blood clock: all blood samples belong to the training set, while all other tissues to the test set. Liver clock: only test samples are shown.
On the liver test samples, MAE was 69 days for WLMT and 58 days for YOMT. There were two reasons for a comparatively good performance of YOMT on these samples: first, the dataset included several samples used for training YOMT, and second, YOMT itself was heavily based on liver samples (44% of all samples used for training YOMT were liver samples), unlike WLMT (23% liver samples). Unlike other DNAm-based age predictors discussed here, the liver clock was based on the methylation levels at dinucleotide positions. We used the coordinates of CpG sites contributing to the liver clock (Wang et al., 2017) as published without modifications. As in the case of the blood methylation clock, the liver-based predictor performed better than WLMT (Figure 3-figure supplement 1) on the samples which were not used in the original training sets of any of the clocks (MAE was 65 days and 244 days, respectively,
It is important to note that obtaining adequate results for the application of YOMT clock was challenging without the original training set. The obtained DNAm ages ranged from 88.2 to 149.5 days, while the actual range of chronological ages of samples was between 7 and 1050 days, and overall correlation between chronological and DNAm ages was relatively low (R2 = 0.36). This result was obtained for the samples where more than 90% of clock CpG sites were covered. Thus, it cannot be explained by insufficient site representation (Supplementary file 1). This led to a situation where YOMT clock showed good precision on samples with chronological ages ~ 3–5 months old, but lost robustness when applied to samples with more advanced chronological ages (Figure 3D). We also noticed and corrected a shift in the coordinates of clock CpG sites defining the blood methylation clock (Petkovich et al., 2017) (Supplementary file 3), wherein the coordinates were accidently shifted to the next CpG site in a reported supplementary table (Petkovich et al., 2017), but not in the analyses. Coordinates of all four clocks used in the current study are reported in Supplementary file 3, which can be used for comparative analyses and further applications.
We have also performed a correlation analysis of the estimated methylation ages obtained from the clocks. For this analysis, we only took samples not used in any training set of the studied clocks and having more than 90% of clock CpG sites covered. WLMT correlated best with the blood clock when applied to the blood samples with
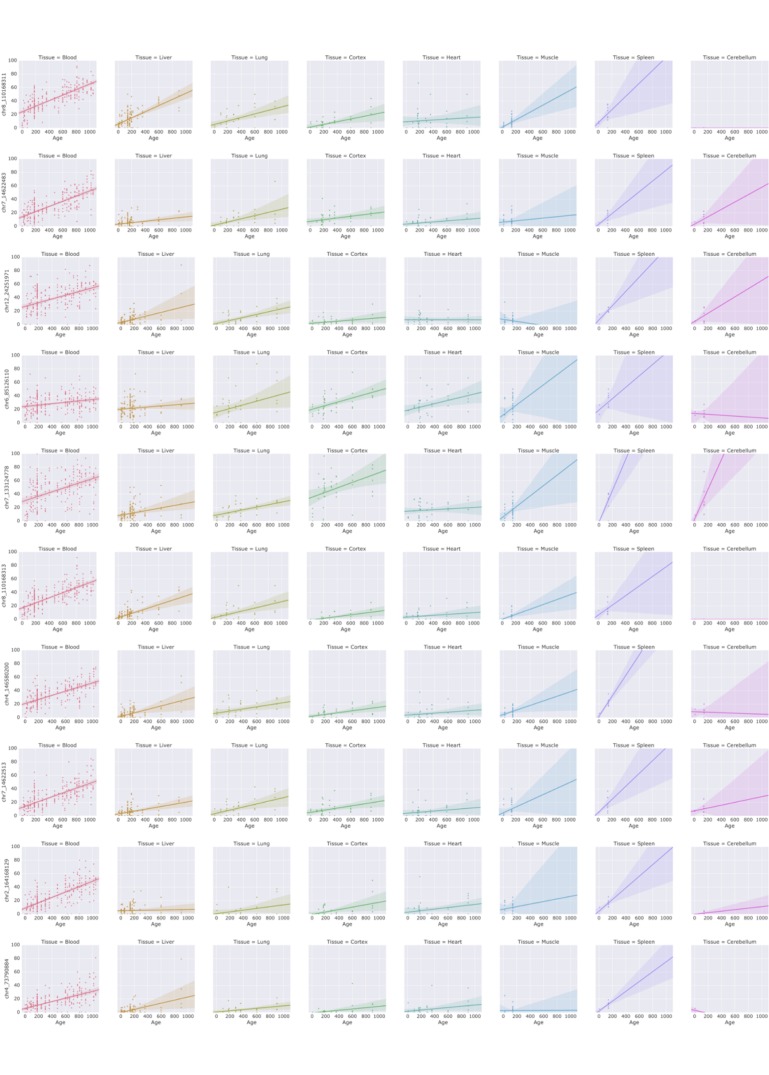
In addition, we analyzed 31 heart, lung and hippocampus samples from an independent dataset (Reizel et al., 2018) (Figure 4). WLMT showed a slightly better performance than the other clocks, with MAE = 53 days while it was 58 days for YOMT, 54 days for the liver clock and 64 days for the blood clock. Interestingly, YOMT and liver clocks produced tight clusters of methylation age points (with standard deviation for YOMT as low as 2 days for young and 3 days for older samples; for the liver clock it was 2 and 8 days, respectively) barely reflecting age change forcing us to conclude that MAE may not be a reliable assessment in this case. To further investigate the comparative behavior of different clocks, we performed linear regression of residual methylation age values against chronological age. Lack of significant slope in this case would be a signature of a clock which does not produce a systematic error and it will be equivalent to having a slope equal to 1 in the linear regression of DNAm age against chronological age. Linear regressions for the residuals in YOMT, blood and liver clocks showed a significant shift of slope from 0. For the WLMT this change was smaller and not significant.
An additional dataset (Reizel et al., 2018) was tested with the four clocks. Solid line represents a perfect correspondence between DNAm age and chronological age. Dashed lines show slopes of linear regressions made for each DNAm clock based on the age values estimated with it. Slopes: 0.14 (YOMT), 0.16 (liver), 1.24 (WLMT), 0.45 (blood). p-values:
Application of DNAm age estimators to known lifespan-modulating interventions
We have applied the four mouse DNAm predictors to detect changes in DNAm age associated with the application of longevity interventions (Figure 5A-F). As previously reported (Petkovich et al., 2017), the blood-based methylation clock reflected the effect of several known lifespan-extending interventions when applied to blood samples (Figure 5A,B,D,E), whereas both YOMT and liver-based methylation clocks did not show a significant DNAm age-modulating effect due to the same interventions. WLMT revealed a shift of average DNAm age in all four cases, but with the given sample size, it was statistically significant only for samples with growth hormone receptor knockout (GRH KO,
Untreated and wild-type samples are shown in green, and intervention samples in orange. (A) Effect of caloric restriction in mouse strain C57BL/6, DNA from blood. (B) Effect of caloric restriction, mouse strain B6D2F1, DNA from blood. (C) Conversion of fibroblasts to iPSCs, DNA from cultured cells. (D) Growth hormone receptor knockout, DNA from blood. (E) Snell dwarf mice, DNA from blood. (F) Ames dwarf mice, DNA from liver. ΔAge was defined as the difference between the DNAm age and the chronological age. *-
WLMT clock sites
The majority of the WLMT clock CpG sites (58%) belong to open sea, 5% to shelves, 11% to shore regions of the genome, and 26% are located within CpG islands. Since we were interested in a common sex-independent signature of aging, the sex chromosomes were excluded from the analysis. Interestingly, all autosomes were represented in the WLMT clock (Figure 2—figure supplement 1). Similarly, clock CpG sites were located in all or almost all (in the blood clock) chromosomes (Figure 2—figure supplement 2).
We found that none of the WLMT clock sites overlapped with the clock sites of YOMT. The overlaps between blood and WLMT and between liver and YOMT clocks were not statistically significant (Figure 2A). However, there was a significant (
Across different genes in the mouse genome, Kcns1 contained clock CpG sites present in both WLMT and blood DNAm clocks; this gene was represented by multiple CpG sites: 6 in the blood clock and 11 in the WLMT. It has been shown recently by pyrosequencing that the CpG sites of this genomic region have a high correlation with age (Han et al., 2018). Interestingly, CpG sites with higher weights on the methylation clock based on RRBS also showed higher correlation with age measured with pyrosequencing. For example, the CpG site located at the position chr2:164168131 had the highest weight in the WLMT clock in this region and was also among the top 5% of the most important CpG sites representing this clock; methylation level on this CpG site was also strongly correlated with age with
To study robustness of the WLMT clock, we have also developed 100 new individual clocks based on the same set of samples used for WLMT construction. To construct every individual clock among 100, this set was split differently into 80% training and 20% test subsets with stratification based on tissues. Thus, the procedure was the same, but samples in the training and test sets varied. Next, we calculated how many times each of the 435 WLMT clock sites appeared in the newly constructed 100 clocks (Figure 2-figure supplement 3A). Remarkably, the five sites which are found in both WLMT and blood clocks were significantly more represented than the other sites (
Overall, we found that single-tissue-based clocks generally reflected tissue-specific age-dependent changes in DNA methylation and were simultaneously more precise when applied to the corresponding tissue samples than multi-tissue clocks. At the same time, while multi-tissue clocks were biased toward tissues better represented in the training sets, they nonetheless better reflected systemic aging. In this regard, both tissue-specific and multi-tissue DNAm clocks should find application in future studies.
Discussion
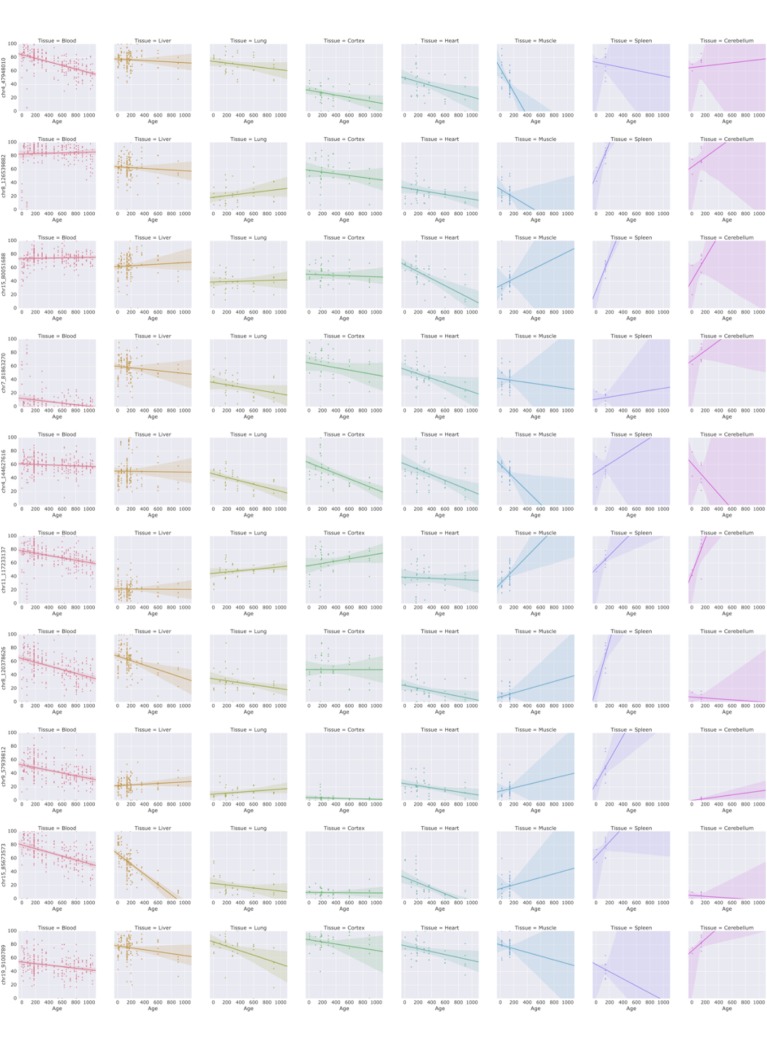
Most of the CpG sites with positive weight in WLMT had low DNAm levels in early life and increased with age (Figure 2—figure supplement 4), and the opposite was true for the clock sites with negative weight (Figure 2—figure supplement 5). A similar pattern of DNAm drifting towards intermediate methylation states was shown for DNAm remodeling during aging (Sziráki et al., 2018). Taken together, these observations support the idea of the DNAm clock being a representation of some global, systemic age-dependent changes (Horvath and Raj, 2018) in DNA methylation rather than being a tool to identify a small set of key genomic positions, methylation status of which determines the methylation age.
DNA methylation is a relatively new evolutionary mechanism to control gene expression. Chordates lack CpG islands (CGI), fishes and amphibians have only a small fraction of transcription start sites (TSS) containing CGI, and most of the TSS are associated with CGIs in warm-blooded vertebrates (Sharif et al., 2010). DNA methylation patterns also show strong tissue specificity (Lokk et al., 2014). As a consequence, DNA methylation profiles change with age differently in individual tissues (Maegawa et al., 2010). Thus, construction of a multi-tissue DNA methylation clock cannot be reduced to a simple superposition of tissue-specific clocks, signifying the presence of a general pattern of aging in the DNA methylome. Furthermore, most CpG sites belonging to tissue-specific DNAm clocks do not contribute to the constructed multi-tissue clock.
Even though DNAm clocks presently seem to be the best available estimators of age, the downside of these tools is that they are not universal and must be recreated or at least adjusted for individual species. Interestingly, this technique was first developed for human samples, whereas its application to model organisms lagged. When the first multi-tissue DNAm clock was developed (Horvath, 2013), thousands of samples processed with the same technology were already available, and the original human multi-tissue clock was built based on 8000 samples representing 82 datasets (Horvath, 2013).
The situation in mice has been and remains drastically different. First, the number of samples sequenced and available in the public domain is lower by more than an order of magnitude. Second, these samples originate from a limited number of studies (Petkovich et al., 2017), and the associated methylomes are thus subject to strong batch effects, which are hard to separate from the tissue-specific effects. Having additional sources of data to construct DNAm clocks would have helped to deal with the biases introduced by individual laboratories and studies. Third, even though all mouse samples have been bisulfite sequenced, the actual experimental protocols used in different studies varied significantly. Performance of mouse clocks is expected to improve with the increase in the number of bisulfite sequenced mouse samples and the addition of new publicly available datasets. However, even the already available clocks can be used to examine the effects of candidate lifespan-extending interventions and address diverse questions in aging research.
Methylation clocks are based on chronological ages of samples. Which of them can help estimate biological age requires further analyses. Testing if the clocks predict survival differences is also a desirable future project, but it could not have been done with the currently existing data. On the other hand, some interventions seem to affect biological age. The ability of the clocks to reflect these interventions would support the idea that methylation clocks may measure biological ages. Besides, clocks with better performance on control samples are better in detecting the effects of interventions. It can be seen for the blood clock: it has the lowest MAE among all clocks on the untreated wild type blood samples (Figure 3E). At the same time, it can detect significant changes in lifespan-extending interventions even in the cases where the other clocks cannot do it with the given sample size (Figure 5A,B,E).
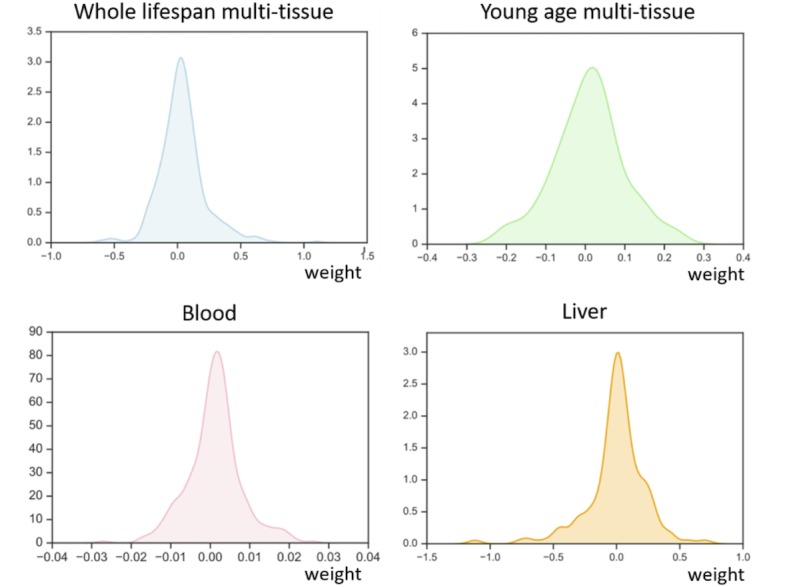
The existing mouse clocks vary in the tissues they are based on as well as in the models that convert weighted DNAm levels to methylation age. WLMT and liver clocks should be used as linear models, while YOMT and blood clock used a different approach, when DNAm data is first converted into a DNAm score, which then requires an additional transformation to reflect age (Supplementary file 3). Another difference between the clocks is the way age was integrated into the models. Chronological age was directly used to construct the WLMT clock, while log-transformed chronological age was used in models based on blood, liver and YOMT, making these clocks more tuned towards young ages. The distributions of weights look similar for all four methylation clocks (Figure 2-figure supplement 6), with weight distributions being zero-symmetric (
The most popular method to measure DNA methylation at many loci, RRBS, is an enrichment method. One of its downsides is the fact that some sites covered in one sample might not be covered at all in another. We directly faced this issue in the present work and, even though only samples with more than 2 million sites covered were selected for training the WLMT clock, these 2 million sites represented mere 10% of all possible CpG sites covered in at least one sample. This brings a question about how to best apply RRBS-based clocks to future samples. The median number of WLMT clock sites covered in the samples not used for the construction of the clock (and, thus, not pre-selected) was 435, equal to the total number of the sites the WLMT clock is based on. The testing set included the independent dataset (Reizel et al., 2018) and intervention tests but not the dataset with a low number of covered sites (Schillebeeckx et al., 2013). None of the clocks comparatively studied here had good clock site coverage in this low-coverage dataset, but WLMT was leading with 75% of the clock sites utilized. Less than 90% of WLMT sites were covered in all WGBS datasets. The same is true for YOMT and blood clock – the other two clocks purely based on RRBS technology. Interestingly, blood clock sites were less represented in the datasets which were not used for training the blood clock. The blood clock is the only one among studied here for which the median site coverage in the independent dataset (Reizel et al., 2018) was below 100% (87 out of 90 sites). This can be explained by the fact that the blood clock was trained on the dataset created under the same conditions, while the other clocks were trained on the overlap of multiple datasets, thus neglecting CpGs in the positions which have experiment-to-experiment variability.
Comparing the clocks, we noticed an interesting phenomenon – both multi-tissue clocks and the blood clock reflect changes of methylation age in relation to changes in chronological age in all tested tissues, even though those changes are detected better in some tissues than in the others. Performance of the blood clock decreases in following order: liver, lung, heart and cortex. But this is not the case for the liver clock, another single tissue-based clock. While it performs well on the liver samples, it fails to reflect changes in any other tissue (Figure 3—figure supplement 2). A possible explanation for this difference can be based on the fact that blood has a better representation of multiple cell types than liver mostly consisting of one cell type – hepatocytes which are polypoid (and as such the majority of liver DNA is the hepatocyte DNA (Duncan et al., 2010)). It is also possible that blood better represents organismal aging than liver.
Overall, we created an easily applicable tool to estimate the DNAm age of mouse samples of different tissues, which works across the entire lifespan of these animals. The constructed multi-tissue DNAm clock can also be applied to mice of both sexes and may be a preferred method to study aging of tissues lacking tissue-specific clocks.
Materials and methods
Datasets
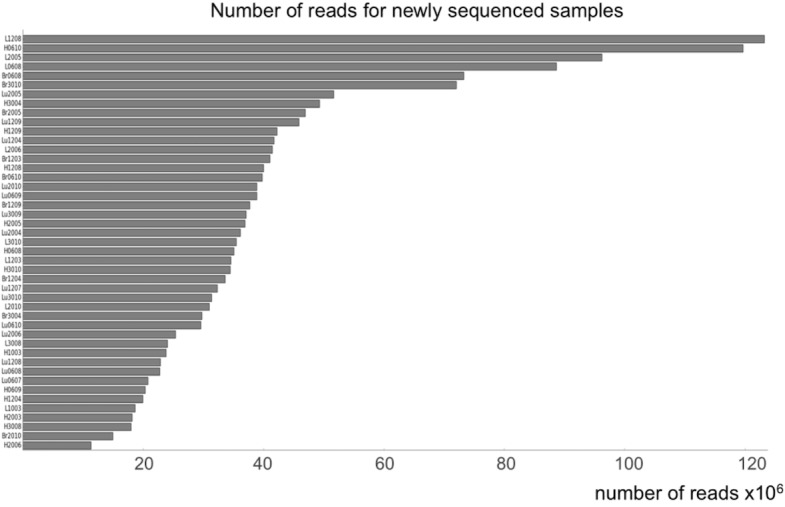
We used tissues from the animals analyzed in our previous study (Petkovich et al., 2017) (C57BL/6N mice; RRID: MGI:5913915). To minimize the effect of brain heterogeneity (Lister et al., 2013), we homogenized the right halves of cortex prior to sampling. For liver, heart and lung sample, we cut 3–10 mg pieces while preserving frozen tissues. DNA was extracted using DNeasy Blood and Tissue Kit from Qiagen and eluted in 200 μl of 10 mM Tris-HCl buffer, pH 8.0. Then, 2 μl of RNase A (Life Technologies) was added to each sample. Samples were incubated at room temperature for 5 min, and DNA was prepared by using Genomic DNA Clean and Concentrator-10 (Zymo). DNA was eluted in 50 μl of TE buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.0) and quantified using a Qubit 2.0 (Life Technologies). RRBS sample preparation was performed using 100 ng of DNA following the protocol from Petkovich et al. (2017). Each library was made of 3–6 samples. In order to avoid age- or tissue-related batch effect, we grouped samples into the libraries distributing each group of samples into different libraries (Supplementary file 5). The libraries were sequenced with Illumina HiSeq2500, PE150 with average 44M (Figure 1-figure supplement 4) reads per sample. 20% of mouse genomic DNA library was spiked in to compensate for low complexity of the libraries. The data obtained in this study were deposited to GEO with the accession number {"type":"entrez-geo","attrs":{"text":"GSE121141","term_id":"121141"}}GSE121141.
Data processing
In addition to data generated by our laboratory, we downloaded publicly available datasets of mouse DNAm profiles, including {"type":"entrez-geo","attrs":{"text":"GSE93957","term_id":"93957"}}GSE93957, {"type":"entrez-geo","attrs":{"text":"GSE60012","term_id":"60012"}}GSE60012, {"type":"entrez-geo","attrs":{"text":"GSE52266","term_id":"52266"}}GSE52266, {"type":"entrez-geo","attrs":{"text":"GSE80761","term_id":"80761"}}GSE80761, {"type":"entrez-geo","attrs":{"text":"GSE45361","term_id":"45361"}}GSE45361, SRP069120, {"type":"entrez-geo","attrs":{"text":"GSE89273","term_id":"89273"}}GSE89273, {"type":"entrez-geo","attrs":{"text":"GSE89274","term_id":"89274"}}GSE89274, {"type":"entrez-geo","attrs":{"text":"GSE84573","term_id":"84573"}}GSE84573, {"type":"entrez-geo","attrs":{"text":"GSE70538","term_id":"70538"}}GSE70538, {"type":"entrez-geo","attrs":{"text":"GSE80672","term_id":"80672"}}GSE80672. Since data in these projects were processed differently, which could in principle affect estimated methylation levels, we utilized raw reads and applied the same pipeline to all samples. The reads were trimmed using Trim Galore v0.4.1 (RRID: SCR_011847) with the parameter --rrbs for all samples, --paired for the paired-end sequenced. For the samples from {"type":"entrez-geo","attrs":{"text":"GSE93957","term_id":"93957"}}GSE93957, we have also added – three_prime_clip_R1 15 –three_prime_clip_R2 15 options to remove unique molecular indices. The filtered reads were mapped to the mouse genome (GRCm38/mm10) using Bismark v0.15.0 (RRID: SCR_005604) with the default parameters to obtain methylation levels and coverage per site. Sites in the mitochondrial genome and sex chromosomes were removed. The same pipeline was applied to the independent dataset {"type":"entrez-geo","attrs":{"text":"GSE85251","term_id":"85251"}}GSE85251.
DNAm clock construction
We have created a dataset of 416 samples which met the following conditions: (1) all included mice were wild-type animals, (2) untreated and not used as an intervention control, (3) number of CpG sites covered by sequencing in every sample was greater than 2 × 106, and (4) number of the overlapping sites in all samples was greater than 100,000. We applied a soft coverage cutoff, excluding CpG sites which had <5x coverage in more than 90% of samples. This resulted in obtaining 193,439 CpG sites included in all DNA methylomes. Custom scripts for data filtering and processing were written in R and python programming languages. They were deposited to GitHub under GNU General Public License: https://github.com/gr-meer/WLMT (Meer, 2018; copy archived at https://github.com/elifesciences-publications/WLMT). The relation of CpG sites to CpG islands and genes was annotated using annotatr package from Bioconductor (Cavalcante and Sartor, 2017) (RRID: SCR_006442). ElasticNet regression was performed using the python scikit-learn package (Pedregosa, 2012) (RRID: SCR_002577). Stratification based on tissue types was applied to splitting samples into training (80%) and test (20%) set and during 10-fold cross validation. Venn diagram was created using Venny 2.1 (http://bioinfogp.cnb.csic.es/tools/venny/).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Matt Kaeberlein, University of Washington, United States.
Jessica K Tyler, Weill Cornell Medicine, United States.
Funding Information
This paper was supported by the following grants:
- National Institute on Aging AG021518 to Vadim N Gladyshev.
- National Institute on Aging AG047200 to Vadim N Gladyshev.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Resources, Data curation, Formal analysis, Validation, Investigation, Writing—original draft, Writing—review and editing.
Conceptualization, Methodology, Writing—review and editing.
Formal analysis, Investigation, Methodology, Writing—review and editing.
Conceptualization, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Additional files
Supplementary file 1.
Sample information.Supplementary file 2.
Information on samples sequenced by RRBS in the study and included in the analysis.Supplementary file 3.
Mouse DNAm clocks.Supplementary file 4.
Genes overlapping in pairs of clocks.Supplementary file 5.
Distribution of samples across libraries.Data availability
Sequencing data have been deposited in GEO under accession code {"type":"entrez-geo","attrs":{"text":"GSE121141","term_id":"121141"}}GSE121141
The following dataset was generated:
Meer MV, Podolskiy DI, Tyshkovskiy AE, Gladyshev VN. 2018. DNA methylation of mouse tissues. NCBI Gene Expression Omnibus. GSE121141
The following previously published datasets were used:
Stubbs TM, von Meyenn F, Katrien-Stark A, Krueger F, Reik W. 2017. Multi-tissue DNA methylation age predictor in mouse. GEO. GSE93957
Reizel Y, Spiro A, Sabag O, Skversky Y, Hecht M, Keshet I, Berman BP, Cedar H. 2014. Gender-specific post-natal demethylation and establishment of epigenetic memory. GEO. GSE60012
Petkovich DA, Podolskiy DI, Lobanov AV, Gladyshev VN. 2016. Using DNA methylation profiling to evaluate biological age and longevity interventions. GEO. GSE80672
Cannon MV, Buchner DA, Hester J, Miller H, Sehayek E, Nadeau JH, Serre D. 2013. Maternal Nutrition Induces Pervasive Gene Expression Changes but no Detectable DNA Methylation Differences in the Liver of Adult Offspring [RRBS] GEO. GSE52266
Zhang C, Edepli KS, Lujambio A. 2016. Genome-wide DNA methylation profiling of mouse liver. GEO. GSE80761
Schillebeeckx M, Mitra RD. 2013. Laser Capture Microdissection-Reduced Representation Bisulfite Sequencing (LCM-RRBS) maps changes in DNA methylation associated with gonadectomy-induced adrenocortical neoplasia in the mouse. GEO. GSE45361
Cole JJ, Robertson NA, Rather MI, Adams PD. 2016. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions (WGBS 1) GEO. GSE89273
Cole JJ, Robertson NA, Rather MI, Adams PD. 2016. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions (WGBS 2) GEO. GSE89274
McCormick H, Hur s, Young P, Cropley J, Booher K, Cheung H, Giannolatou E, Suter C. 2016. Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. GEO. GSE84573
References
- Bocklandt S, Lin W, Sehl ME, Sánchez FJ, Sinsheimer JS, Horvath S, Vilain E. Epigenetic predictor of age. PLOS ONE. 2011;6:e14821. doi: 10.1371/journal.pone.0014821. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cavalcante RG, Sartor MA. annotatr: genomic regions in context. Bioinformatics. 2017;33:2381–2383. doi: 10.1093/bioinformatics/btx183. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Cole JJ, Robertson NA, Rather MI, Thomson JP, McBryan T, Sproul D, Wang T, Brock C, Clark W, Ideker T, Meehan RR, Miller RA, Brown-Borg HM, Adams PD. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biology. 2017;18:58. doi: 10.1186/s13059-017-1185-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- de Magalhães JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Dollé ME, Snyder WK, Gossen JA, Lohman PH, Vijg J. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. PNAS. 2000;97:8403–8408. doi: 10.1073/pnas.97.15.8403. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, Finegold MJ, Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hahn O, Grönke S, Stubbs TM, Ficz G, Hendrich O, Krueger F, Andrews S, Zhang Q, Wakelam MJ, Beyer A, Reik W, Partridge L. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biology. 2017;18:56. doi: 10.1186/s13059-017-1187-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Han Y, Eipel M, Franzen J, Sakk V, Dethmers-Ausema B, Yndriago L, Izeta A, de Haan G, Geiger H, Wagner W. Epigenetic age-predictor for mice based on three CpG sites. eLife. 2018;7:e37462. doi: 10.7554/eLife.37462. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Molecular Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [PubMed] [CrossRef] [Google Scholar]
- Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C, Downey SL, Johnson BE, Fouse SD, Delaney A, Zhao Y, Olshen A, Ballinger T, Zhou X, Forsberg KJ, Gu J, Echipare L, O'Geen H, Lister R, Pelizzola M, Xi Y, Epstein CB, Bernstein BE, Hawkins RD, Ren B, Chung WY, Gu H, Bock C, Gnirke A, Zhang MQ, Haussler D, Ecker JR, Li W, Farnham PJ, Waterland RA, Meissner A, Marra MA, Hirst M, Milosavljevic A, Costello JF. Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications. Nature Biotechnology. 2010;28:1097–1105. doi: 10.1038/nbt.1682. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biology. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [PubMed] [CrossRef] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–591. doi: 10.18632/aging.101414. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341:1237905. doi: 10.1126/science.1237905. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Lokk K, Modhukur V, Rajashekar B, Märtens K, Mägi R, Kolde R, Koltšina M, Nilsson TK, Vilo J, Salumets A, Tõnisson N. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biology. 2014;15:r54. doi: 10.1186/gb-2014-15-4-r54. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, Zhang N, Liang S, Donehower LA, Issa JP. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Research. 2010;20:332–340. doi: 10.1101/gr.096826.109. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Maierhofer A, Flunkert J, Oshima J, Martin GM, Haaf T, Horvath S. Accelerated epigenetic aging in Werner syndrome. Aging. 2017;9:1143–1152. doi: 10.18632/aging.101217. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Meer MV. gr-meer/WLMT. 3GitHub. 2018 https://github.com/gr-meer/WLMT
- Pedregosa F. Scikit-learn: Machine learning in python. Journal of Machine Learning Research : JMLR. 2012;12:2825–2830. [Google Scholar]
- Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metabolism. 2017;25:954–960. doi: 10.1016/j.cmet.2017.03.016. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Podolskiy DI, Gladyshev VN. Intrinsic Versus Extrinsic Cancer Risk Factors and Aging. Trends in Molecular Medicine. 2016;22:833–834. doi: 10.1016/j.molmed.2016.08.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Podolskiy DI, Lobanov AV, Kryukov GV, Gladyshev VN. Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nature Communications. 2016;7:12157. doi: 10.1038/ncomms12157. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Polanowski AM, Robbins J, Chandler D, Jarman SN. Epigenetic estimation of age in humpback whales. Molecular Ecology Resources. 2014;14:976–987. doi: 10.1111/1755-0998.12247. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, Ritz B, Bandinelli S, Neuhouser ML, Beasley JM, Snetselaar L, Wallace RB, Tsao PS, Absher D, Assimes TL, Stewart JD, Li Y, Hou L, Baccarelli AA, Whitsel EA, Horvath S. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–446. doi: 10.18632/aging.101168. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Reizel Y, Sabag O, Skversky Y, Spiro A, Steinberg B, Bernstein D, Wang A, Kieckhaefer J, Li C, Pikarsky E, Levin-Klein R, Goren A, Rajewsky K, Kaestner KH, Cedar H. Postnatal DNA demethylation and its role in tissue maturation. Nature Communications. 2018;9:2040. doi: 10.1038/s41467-018-04456-6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Schillebeeckx M, Schrade A, Löbs AK, Pihlajoki M, Wilson DB, Mitra RD. Laser capture microdissection-reduced representation bisulfite sequencing (LCM-RRBS) maps changes in DNA methylation associated with gonadectomy-induced adrenocortical neoplasia in the mouse. Nucleic Acids Research. 2013;41:e116. doi: 10.1093/nar/gkt230. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sharif J, Endo TA, Toyoda T, Koseki H. Divergence of CpG island promoters: a consequence or cause of evolution? Development, Growth & Differentiation. 2010;52:545–554. doi: 10.1111/j.1440-169X.2010.01193.x. [PubMed] [CrossRef] [Google Scholar]
- Stubbs TM, Bonder MJ, Stark AK, Krueger F, von Meyenn F, Stegle O, Reik W, BI Ageing Clock Team Multi-tissue DNA methylation age predictor in mouse. Genome Biology. 2017;18:68. doi: 10.1186/s13059-017-1203-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Sziráki A, Tyshkovskiy A, Gladyshev VN. Global remodeling of the mouse DNA methylome during aging and in response to calorie restriction. Aging Cell. 2018;17:e12738. doi: 10.1111/acel.12738. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Thompson MJ, vonHoldt B, Horvath S, Pellegrini M. An epigenetic aging clock for dogs and wolves. Aging. 2017;9:1055–1068. doi: 10.18632/aging.101211. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Wang T, Tsui B, Kreisberg JF, Robertson NA, Gross AM, Yu MK, Carter H, Brown-Borg HM, Adams PD, Ideker T. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biology. 2017;18:57. doi: 10.1186/s13059-017-1186-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zubakov D, Liu F, van Zelm MC, Vermeulen J, Oostra BA, van Duijn CM, Driessen GJ, van Dongen JJ, Kayser M, Langerak AW. Estimating human age from T-cell DNA rearrangements. Current Biology. 2010;20:R970–R971. doi: 10.1016/j.cub.2010.10.022. [PubMed] [CrossRef] [Google Scholar]
- eLife. 2018; 7: e40675. »
- Decision letter
Decision letter
In the interests of transparency, eLife includes the editorial decision letter and accompanying author responses. A lightly edited version of the letter sent to the authors after peer review is shown, indicating the most substantive concerns; minor comments are not usually included.
Thank you for submitting your article "A whole lifespan mouse multi-tissue DNA methylation clock" for consideration by eLife. Your article has been reviewed by three peer reviewers, and the evaluation has been overseen by a Reviewing Editor and Jessica Tyler as the Senior Editor. The following individual involved in review of your submission has agreed to reveal his identity: Peter Adams (Reviewer #2).
The reviewers have discussed the reviews with one another and the Reviewing Editor has drafted this decision to help you prepare a revised submission.
Summary:
The reviewers appreciated the value of the whole lifespan mouse multi-tissue DNA methylation clock (WLMT clock) and agree in principle that this is suitable as an eLife Tools and Resources article. Some concerns were raised however, and after consultation, we have identified several places where the manuscript could be improved. Most notably, all three reviewers felt that it is important to show how well the WLMT clock performs on additional data sets that have not been pre-selected to include all of the clock CpGs. The authors also must confirm that the raw data have been deposited at GEO and provide the GEO ID or a reviewer access link.
Essential Revisions:
1) While the WLMT clock performs well on samples from the test and training sets the precision appears to be much lower on other samples. This is probably due to the fact that not all of the relevant 435 clock sites are covered in these completely independent samples (while the test set was preselected to cover all 435 sites). The authors should assess how the WLMT performs on completely independent datasets. Furthermore, the authors should describe how to adjust for missing clock sites. Otherwise the method won't be useful for other researchers to apply to new datasets. Perhaps some new datasets could be generated or publicly available datasets should be used to address this important issue.
2) A major question about the novel clock is how it differs from other clocks. There are a couple of additional analyses that should be performed to address this important question.
a) The authors should compute correlations among the different epigenetic clocks and also among their age-acceleration-residuals/delta-age values (i.e. the difference between epigenetic age and chronological age).
b) Comparison of the WLMT clock with other clocks was done with different subsets of samples since almost all samples turned out to be included into one or another clock training set. However, for a fair comparison only completely independent datasets from different tissues should be used.
3) The most important results in the paper are contained in Figure 4, and these are not very easy to interpret from the graph or the accompanying text. Most interest in epigenetic clocks is driven by the hypothesis that differences between epigenetic age and chronological age (called "delta-age" or "epigenetic age acceleration residual", depending on how computed) reflect processes of biological aging. The authors present tests of this hypothesis in Figure 4 by comparing delta-age values of mice exposed to different longevity-enhancing or mortality-risk-increasing interventions. The result of this analysis is that the blood-based clock often shows differences in delta-age values, whereas results are less consistent for other clocks. This result is not transparent from the figure nor is it all that transparent from the text. It needs to be clarified. For example, it would be easier to see the result if the intervention and control group data for each clock were presented side by side so the visual comparison suggested by the figure matched the statistical analysis performed.
4) There are several places where it was felt that the authors overstate or overinterpret their results. Please correct these as suggested:
a) The authors state that Figure 3A is "is biased towards a better performance of the other clocks because the biological ages of a combination of their test and training samples are estimated, thus artificially improving apparent performance", whereas WLMT is only measured against its test set (not its training set). I think this may underestimate the inter-lab qualitative variations in RRBS datasets. One reason the human clocks work so well across datasets is, I think, the high technical consistency of the Illumina arrays between labs. WLMT is compared to RRBS data generated largely in the same lab, while the other datasets are compared to WLMT data generated in a different lab. The inter-lab comparisons my bias against the other clocks due to qualitative variations in RRBS data between labs. RRBS in different labs likely has different coverage of different CpGs, for example. Hence, I think this statement should be moderated.
b) The authors state "DNAm clocks remain the most precise markers of biological age". I think here the authors mean "chronological" age not "biological" age. First, in the Introduction the authors support this statement by reference to measures of chronological age, not biological age. Second, there is no consensus as to how to best measure biological age, so it is an overstatement to say that DNAm clocks are the best. Chronological and biological age are not the same and the terms should not be used interchangeably.
c) The statement that "DNA methylation is a relatively new evolutionary mechanism to control gene expression; among animals, it appears in vertebrates […]" (Discussion paragraph two) is not covered by Lokk et al., 2014 and it might not be correct.
d) The authors indicated that "the constructed multi tissue DNAm clock can also be applied to mice of different genetic backgrounds" (Discussion final paragraph), but this has not been systematically analyzed in this study.
e) The authors assert their multi-tissue clock is unique from previous tissue specific clocks partly on the basis that few of the CpGs included in the multi-tissue clock are included in tissue-specific clocks (Discussion paragraph two). The elastic net run on the same data against the same criterion with slightly different parameters may select different CpGs from one run to the next. The question is whether the different CpGs are more or less statistically independent of one another. The authors should test this if they wish to make claims about the uniqueness of the CpGs in their multi-tissue clock or the discussion should be appropriately modified.
Contributor Information
Matt Kaeberlein, University of Washington, United States.
Wolfgang Wagner, RWTH Aachen University, Germany.
Peter Adams, Beatson Institute, United Kingdom.
- eLife. 2018; 7: e40675. »
- Author response
Author response
The reviewers appreciated the value of the whole lifespan mouse multi-tissue DNA methylation clock (WLMT clock) and agree in principle that this is suitable as an eLife Tools and Resources article. Some concerns were raised however, and after consultation, we have identified several places where the manuscript could be improved. Most notably, all three reviewers felt that it is important to show how well the WLMT clock performs on additional data sets that have not been pre-selected to include all of the clock CpGs. The authors also must confirm that the raw data have been deposited at GEO and provide the GEO ID or a reviewer access link.
We would like to thank reviewers for the great comments and insights. We carried out additional analyses including the test of the WLMT clock on other publicly available datasets. We provide more details below in response to specific comments. In addition, raw methylation data and derived methylation levels on CpG sites used for training and testing the developed clock have been deposited to GEO database with the accession number {"type":"entrez-geo","attrs":{"text":"GSE121141","term_id":"121141"}}GSE121141.
Essential Revisions:
1) While the WLMT clock performs well on samples from the test and training sets the precision appears to be much lower on other samples. This is probably due to the fact that not all of the relevant 435 clock sites are covered in these completely independent samples (while the test set was preselected to cover all 435 sites). The authors should assess how the WLMT performs on completely independent datasets. Furthermore, the authors should describe how to adjust for missing clock sites. Otherwise the method won't be useful for other researchers to apply to new datasets. Perhaps some new datasets could be generated or publicly available datasets should be used to address this important issue.
We have tested an independent dataset, which included heart, lung and hippocampus samples from 1- and 20-week-old mice. For the WLMT, mean absolute deviance error between methylation age and chronological age was found to be ~53 days. We also tested how well sites contributing to different studied clocks are covered in this dataset. The median coverage was equal to the total number of sites for WLMT, YOMT and liver clocks, and it was 87 (97%) for the blood clock. We included this new data in Figure 4 of the manuscript. In addition, we tested the number of sites covered for different clocks in different datasets. Even though the overlap of all samples included into our dataset was ~10% of the total number of CpG sites covered in each sample, clock sites contributing to any one of four studied clocks had a high coverage (>90%) in RRBS samples with at least 2.106 sites covered. The missing sites can be compensated for by removing them from all samples examined in the analysis. While this may affect the estimated DNAm age of control samples, it ultimately helps to avoid a situation where variations in DNAm age difference are artificially created by difference in coverage of CpGs across different samples. In the case when more than 90% of clock sites are missing, which we observe for WGBS samples, we don’t recommend relying on this clock.
2) A major question about the novel clock is how it differs from other clocks. There are a couple of additional analyses that should be performed to address this important question.
a) The authors should compute correlations among the different epigenetic clocks and also among their age-acceleration-residuals/delta-age values (i.e. the difference between epigenetic age and chronological age).
To investigate how WLMT differs from the other published DNAm clocks we performed an analysis of correlation between methylation ages obtained using these clocks. For this study, we only used samples not included in any of the training sets of the published clocks. Also, more than 90% of the clock sites were covered in every used sample. We found that WLMT correlates best with the blood clock when applied to the blood samples withr=0.90, whiler=0.37, when the clocks are applied to other tissues. Similarly, the correlation coefficient for WLMT and the liver clock dropped from r=0.80on liver samples to 0.13 when applied to the other tissues. At the same time, correlation between WLMT and YOMT had an intermediate value (r=0.57). In addition, we performed a correlation analysis of the delta-age values (i.e. the difference between epigenetic age and chronological age). This analysis showed a pattern which differs from the comparison of the absolute values of the DNAm age. Correlation between WLTM and blood clock was 0.42 and 0.63 on blood and non-blood samples, respectively. Meanwhile, the liver clock correlated with WLMT with r=0.81on liver samples and r=0.63on the other samples. Correlation of the delta values of WLMT and YOMT was slightly better than on the absolute values of these clocks, with r=0.66. We included this information in the revised manuscript.
b) Comparison of the WLMT clock with other clocks was done with different subsets of samples since almost all samples turned out to be included into one or another clock training set. However, for a fair comparison only completely independent datasets from different tissues should be used.
To further compare the clocks, we analyzed a completely independent dataset that included heart, lung and hippocampus samples (Figure 4 of the revised manuscript). The WLMT clock shows slightly better performance than the other clocks overall with MAE=53 days, while MAE is equal to 58 days for YOMT, 54 days for the liver clock and 64 days for the blood clock. Interestingly, YOMT and liver clocks produced tight clusters (with very low deviations of DNAm age in each cluster corresponding to a particular chronological age) barely reflecting age change. Thus, MAE might not be the best tool to compare performance of the clocks in this case. To further investigate the comparative behavior of different clocks, we performed linear regression of estimated DNAm age to the chronological age for all four of them. Linear regressions for YOMT, blood and liver clocks showed a significant shift of slope from 1. For the WLMT this change of slope was smaller and not significant. Slopes of the regressions performed on DNAm age and chronological age were: 0.14 (YOMT), 0.16 (liver), 1.24 (WLMT), 0.45 (blood). P-values: p=1.4⋅10-39(YOMT), p=2.8⋅10-29(liver), (WLMT), (blood). We included these data in the paper.
3) The most important results in the paper are contained in Figure 4, and these are not very easy to interpret from the graph or the accompanying text. Most interest in epigenetic clocks is driven by the hypothesis that differences between epigenetic age and chronological age (called "delta-age" or "epigenetic age acceleration residual", depending on how computed) reflect processes of biological aging. The authors present tests of this hypothesis in Figure 4 by comparing delta-age values of mice exposed to different longevity-enhancing or mortality-risk-increasing interventions. The result of this analysis is that the blood-based clock often shows differences in delta-age values, whereas results are less consistent for other clocks. This result is not transparent from the figure nor is it all that transparent from the text. It needs to be clarified. For example, it would be easier to see the result if the intervention and control group data for each clock were presented side by side so the visual comparison suggested by the figure matched the statistical analysis performed.
We have modified the corresponding figure (Figure 5 in the revised manuscript) by presenting the results for control groups and interventions side by side. We have also expanded the corresponding text, which now reads as follows:
“We have applied the four mouse DNAm predictors to detect changes in DNAm age associated with application of longevity interventions (Figure 5A-F). […] Also, since RRBS-based DNAm clocks don’t have good coverage in WGBS samples, RRBS/WGBS – based liver clock is especially recommended for WGBS analysis performed on liver samples and it should be further tested on non-liver WGBS samples.”
4) There are several places where it was felt that the authors overstate or overinterpret their results. Please correct these as suggested:
a) The authors state that Figure 3A is "is biased towards a better performance of the other clocks because the biological ages of a combination of their test and training samples are estimated, thus artificially improving apparent performance", whereas WLMT is only measured against its test set (not its training set). I think this may underestimate the inter-lab qualitative variations in RRBS datasets. One reason the human clocks work so well across datasets is, I think, the high technical consistency of the Illumina arrays between labs. WLMT is compared to RRBS data generated largely in the same lab, while the other datasets are compared to WLMT data generated in a different lab. The inter-lab comparisons my bias against the other clocks due to qualitative variations in RRBS data between labs. RRBS in different labs likely has different coverage of different CpGs, for example. Hence, I think this statement should be moderated.
We thank the reviewer for the comment. Indeed, variability of results between different labs, researchers and experiments is definitely more of an issue for RRBS-based projects than it is for the Illumina arrays. Following the reviewer’s suggestion, we moderated the sentence: “[…] on one hand is biased towards a better performance of the other clocks due to the fact that the methylation ages of a combination of their test and training samples are estimated, thus artificially improving apparent performance. But on the other hand, WGBS was based on samples representing all these datasets. Since RRBS is affected by where and by whom it was performed, this gives an advantage to WLMT because other clocks were tested on the datasets not represented in their original training sets.”
b) The authors state "DNAm clocks remain the most precise markers of biological age". I think here the authors mean "chronological" age not "biological" age. First, in the Introduction the authors support this statement by reference to measures of chronological age, not biological age. Second, there is no consensus as to how to best measure biological age, so it is an overstatement to say that DNAm clocks are the best. Chronological and biological age are not the same and the terms should not be used interchangeably.
While we believe that DNA methylation age is a particular reflection of a true biological age (taking for example into account the fact that DNA methylation age is reset to nearly 0 on iPS cells), we generally agree with this sentiment and modified the text accordingly, both the sentences mentioned by the reviewers and the other sections of the text throughout the manuscript.
c) The statement that "DNA methylation is a relatively new evolutionary mechanism to control gene expression; among animals, it appears in vertebrates […]" (Discussion paragraph two) is not covered by Lokk et al., 2014 and it might not be correct.
Thank you for catching this error in referencing. We added the missing reference and modified the test. Now it reads as follows: "DNA methylation is a relatively new evolutionary mechanism to control gene expression. Chordates lack CpG islands (CGI), fishes and amphibians have only a small fraction of transcription start sites (TSS) containing CGI, but most of the TSS are associated with CGIs in warm-blooded vertebrates.”
d) The authors indicated that "the constructed multi tissue DNAm clock can also be applied to mice of different genetic backgrounds" (Discussion final paragraph), but this has not been systematically analyzed in this study.
Thank you for pointing this out. We have added a comparison of WLMT performance on male and female samples, showing that there is no significant difference in DNAm age estimated for male and female samples of similar ages (p=0.3, two-tailed Mann–Whitney U test). We didn’t see a significant difference between C57BL6-derived strains (p=0.25), but the number of available RRBS samples of other mouse strains was not sufficient to perform a comprehensive analysis. Thus, we have removed the statement related to the genetic background.
e) The authors assert their multi-tissue clock is unique from previous tissue specific clocks partly on the basis that few of the CpGs included in the multi-tissue clock are included in tissue-specific clocks (Discussion paragraph two). The elastic net run on the same data against the same criterion with slightly different parameters may select different CpGs from one run to the next. The question is whether the different CpGs are more or less statistically independent of one another. The authors should test this if they wish to make claims about the uniqueness of the CpGs in their multi-tissue clock or the discussion should be appropriately modified.
Thank you for the valuable suggestions. In order to test the uniqueness of the clock CpG sites we have additionally constructed 100 clocks using elastic net regression on the same dataset varying the samples included in the training and test sets. The CpGs which overlap between the WLMT and blood clock appeared in significantly larger number of the clocks (p-value =3.6x10-4, Wilcoxon test). At the same time, the vast majority of the sites was observed in less than a half of these new clocks further supporting the concept of the global methylation change during aging, allowing us to select different subsets of CpG sites for DNAm clocks. We included a corresponding figure supplement (Figure 2—figure supplement 3).